Ofejiro Blessing Pereye, Yuko Nakagawa, Takashi Sato, Ayako Fukunaka, Shuhei Aoyama, Yuya Nishida, Wakana Mizutani, Nanami Kobayashi, Yohei Morishita, Tetsunari Oyama, Reika Kawabata-Iwakawa, Hirotaka Watada, Hiroki Mizukami, Akihisa Fukuda, Yoshio Fujitani
下载PDF
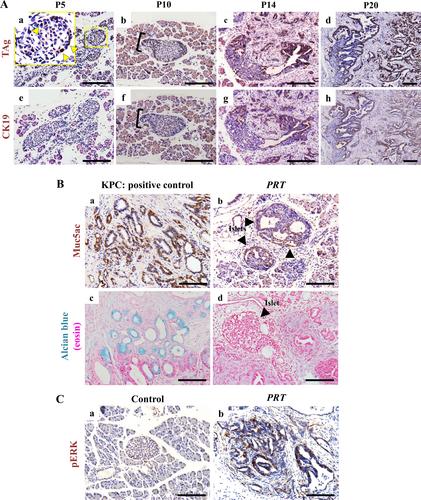
{"title":"确定 Ppy 系细胞是胰腺导管腺癌的新起源。","authors":"Ofejiro Blessing Pereye, Yuko Nakagawa, Takashi Sato, Ayako Fukunaka, Shuhei Aoyama, Yuya Nishida, Wakana Mizutani, Nanami Kobayashi, Yohei Morishita, Tetsunari Oyama, Reika Kawabata-Iwakawa, Hirotaka Watada, Hiroki Mizukami, Akihisa Fukuda, Yoshio Fujitani","doi":"10.1002/path.6295","DOIUrl":null,"url":null,"abstract":"<p>The <i>Ppy</i> gene encodes pancreatic polypeptide (PP) secreted by PP- or γ-cells, which are a subtype of endocrine cells localised mainly in the islet periphery. For a detailed characterisation of PP cells, we aimed to establish PP cell lines. To this end, we generated a mouse model harbouring the SV40 large T antigen (TAg) in the <i>Rosa26</i> locus, which is expressed upon <i>Ppy</i>-promoter-mediated Cre–loxP recombination. Whereas <i>Insulin1</i>-<i>Cre</i>ERT-mediated <i>TAg</i> expression in beta cells resulted in insulinoma, surprisingly, <i>Ppy</i>-<i>Cre</i>-mediated <i>TAg</i> expression resulted in the malignant transformation of <i>Ppy</i>-lineage cells. These mice showed distorted islet structural integrity at 5 days of age compared with normal islets. CK19<sup>+</sup> duct-like lesions contiguous with the islets were observed at 2 weeks of age, and mice developed aggressive pancreatic ductal adenocarcinoma (PDAC) at 4 weeks of age, suggesting that PDAC can originate from the islet/endocrine pancreas. This was unexpected as PDAC is believed to originate from the exocrine pancreas. RNA-sequencing analysis of <i>Ppy</i>-lineage islet cells from 7-day-old <i>TAg</i><sup><i>+</i></sup> mice showed a downregulation and an upregulation of endocrine and exocrine genes, respectively, in addition to the upregulation of genes and pathways associated with PDAC. These results suggest that the expression of an oncogene in <i>Ppy</i>-lineage cells induces a switch from endocrine cell fate to PDAC. Our findings demonstrate that <i>Ppy</i>-lineage cells may be an origin of PDAC and may provide novel insights into the pathogenesis of pancreatic cancer, as well as possible therapeutic strategies. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 4-5","pages":"429-441"},"PeriodicalIF":5.6000,"publicationDate":"2024-06-04","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6295","citationCount":"0","resultStr":"{\"title\":\"Identification of Ppy-lineage cells as a novel origin of pancreatic ductal adenocarcinoma\",\"authors\":\"Ofejiro Blessing Pereye, Yuko Nakagawa, Takashi Sato, Ayako Fukunaka, Shuhei Aoyama, Yuya Nishida, Wakana Mizutani, Nanami Kobayashi, Yohei Morishita, Tetsunari Oyama, Reika Kawabata-Iwakawa, Hirotaka Watada, Hiroki Mizukami, Akihisa Fukuda, Yoshio Fujitani\",\"doi\":\"10.1002/path.6295\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The <i>Ppy</i> gene encodes pancreatic polypeptide (PP) secreted by PP- or γ-cells, which are a subtype of endocrine cells localised mainly in the islet periphery. For a detailed characterisation of PP cells, we aimed to establish PP cell lines. To this end, we generated a mouse model harbouring the SV40 large T antigen (TAg) in the <i>Rosa26</i> locus, which is expressed upon <i>Ppy</i>-promoter-mediated Cre–loxP recombination. Whereas <i>Insulin1</i>-<i>Cre</i>ERT-mediated <i>TAg</i> expression in beta cells resulted in insulinoma, surprisingly, <i>Ppy</i>-<i>Cre</i>-mediated <i>TAg</i> expression resulted in the malignant transformation of <i>Ppy</i>-lineage cells. These mice showed distorted islet structural integrity at 5 days of age compared with normal islets. CK19<sup>+</sup> duct-like lesions contiguous with the islets were observed at 2 weeks of age, and mice developed aggressive pancreatic ductal adenocarcinoma (PDAC) at 4 weeks of age, suggesting that PDAC can originate from the islet/endocrine pancreas. This was unexpected as PDAC is believed to originate from the exocrine pancreas. RNA-sequencing analysis of <i>Ppy</i>-lineage islet cells from 7-day-old <i>TAg</i><sup><i>+</i></sup> mice showed a downregulation and an upregulation of endocrine and exocrine genes, respectively, in addition to the upregulation of genes and pathways associated with PDAC. These results suggest that the expression of an oncogene in <i>Ppy</i>-lineage cells induces a switch from endocrine cell fate to PDAC. Our findings demonstrate that <i>Ppy</i>-lineage cells may be an origin of PDAC and may provide novel insights into the pathogenesis of pancreatic cancer, as well as possible therapeutic strategies. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"263 4-5\",\"pages\":\"429-441\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-06-04\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6295\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6295\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6295","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


