将适当的辅助刺激分子作为溶瘤疱疹病毒的有效载荷,从而将系统生物学与肿瘤内基因治疗相结合
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
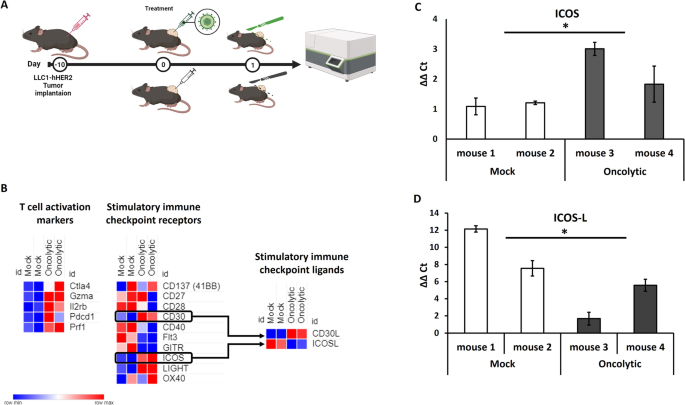
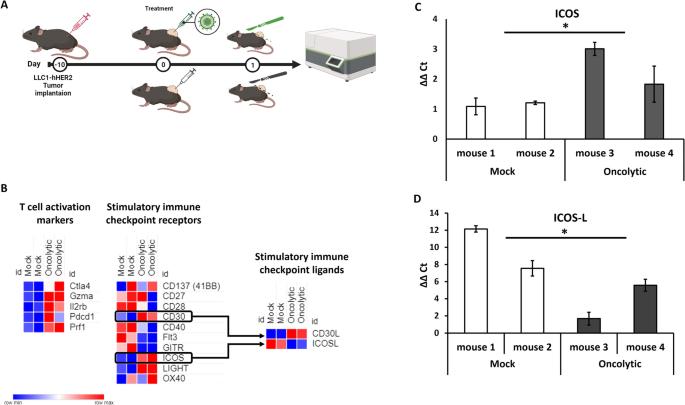
系统生物学已被应用于癌症领域的多尺度层面,改善了癌症预防、诊断并实现了精准医疗方法。虽然系统生物学可以扩展肿瘤治疗的知识和技能,但由于癌症的复杂性、异质性和多样性,它不仅适用于不同的癌症适应症,而且还适用于其在空间和时间上的演变过程,因此它也是一项具有挑战性的任务。在此,我们通过分析溶瘤病毒诱导的肿瘤微环境转录扰动特征,旨在合理设计新型武装溶瘤疱疹病毒。我们发现,HSV-1 的瘤内肿瘤溶解疗法会诱导 T 细胞活化特征,并转录激活几种成本调控分子。我们确定了不同表达的成本刺激受体和结合伙伴,其中可诱导的协同刺激因子(ICOS)可能是最有益的靶向疗法。通过体内外转录组学分析,我们探索了将溶瘤病毒作为一种联合治疗策略的潜力;特别是,我们设计了一种编码 ICOSL 的靶向疱疹病毒(THV_ICOSL),与未武装的亲代病毒相比,它能显著改善肿瘤大小的控制。此外,与 PD-1 抑制剂联用可提高抗肿瘤疗效,这可以通过注射溶瘤病毒后 PD-1 和配体对(PD-L1/PD-L2)的上调来预测。这种病毒的人类版本编码 hICOSL 直向同源物,可通过触发 ICOS 通路有效、特异地激活人类 T 细胞。我们的数据支持以数据为驱动生成武装溶瘤病毒,作为与检查点抑制剂的联合免疫疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Integrating system biology and intratumor gene therapy by trans-complementing the appropriate co-stimulatory molecule as payload in oncolytic herpes virus
Systems biology has been applied at the multi-scale level within the cancer field, improving cancer prevention, diagnosis and enabling precision medicine approaches. While systems biology can expand the knowledge and skills for oncological treatment, it also represents a challenging expedition due to cancer complexity, heterogeneity and diversity not only between different cancer indications, but also in its evolution process through space and time. Here, by characterizing the transcriptional perturbations of the tumor microenvironment induced by oncolytic, we aimed to rationally design a novel armed oncolytic herpes virus. We found that intratumor oncovirotherapy with HSV-1 induces T-cell activation signatures and transcriptionally activates several costimulatory molecules. We identified differentially expressed costimulatory receptors and binding partners, where inducible co-stimulators (ICOS) resulted in the potentially most beneficial targeted therapy. Through an ex-vivo transcriptomic analysis, we explored the potential of arming an oncolytic virus as a combination therapy strategy; in particular, we engineered a targeted herpes virus encoding ICOSL (THV_ICOSL), which resulted in a significant improvement in tumor size control compared to unarmed parental virus. Also, combination with a PD-1 inhibitor enhanced antitumor efficacy as predictable by upregulation of PD-1 and ligands pair (PD-L1/PD-L2) upon oncolytic virus injection. Generation of the human version of this virus encoding hICOSL orthologue effectively and specifically activated human T cells by triggering the ICOS pathway. Our data support the data-driven generation of armed oncolytic viruses as combination immunotherapeutic with checkpoint inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


