TL1A 和 IL-18 协同作用可促进小鼠胸腺粒细胞的 GM-CSF 依赖性生成
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
急性全身性炎症会严重改变免疫系统的功能,通常会促进骨髓造血,而牺牲淋巴造血。在胸腺中,全身性炎症会导致急性胸腺萎缩,从而损害 T 淋巴细胞的生成。除了抑制 T 细胞发育外,全身性炎症影响胸腺的机制仍不清楚。在这里,我们描述了TL1A和IL-18如何协同抑制T淋巴细胞生成以促进胸腺骨髓细胞生成。在病毒诱导的胸腺萎缩感染小鼠巨细胞病毒(MCMV)或小鼠肺炎病毒(PVM)期间,胸腺中这两种细胞因子的蛋白水平升高。体内注射TL1A和IL-18会诱发急性胸腺萎缩,同时胸腺中性粒细胞会增大。用Ms4a3-Cre小鼠绘制的命运图谱显示,胸腺中性粒细胞是从胸腺粒细胞-单核细胞祖细胞(GMPs)中产生的,而Rag1-Cre命运图谱则显示了与淋巴细胞共同的发育路径。这些效应可通过新生儿胸腺器官培养物(NTOCs)进行体内外模拟,其中TL1A和IL-18协同增强了中性粒细胞的产生和排出。LY411575抑制剂阻断NOTCH可增加培养物中中性粒细胞的数量,这表明NOTCH限制了稳态胸腺粒细胞生成。为促进骨髓造血,TL1A 和 IL-18 协同提高了 NTOC 中 GM-CSF 的水平,而 GM-CSF 主要由胸腺 ILC1s 产生。此外,TL1A和IL-18诱导的粒细胞生成在Csf2rb-/-小鼠的NTOC中和GM-CSFR抗体阻断后完全被阻止,这表明GM-CSF是驱动胸腺粒细胞生成的重要因子。综上所述,我们的研究结果表明,TL1A和IL-18协同诱导急性胸腺萎缩,同时以NOTCH和GM-CSF控制的方式促进髓外胸腺造粒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
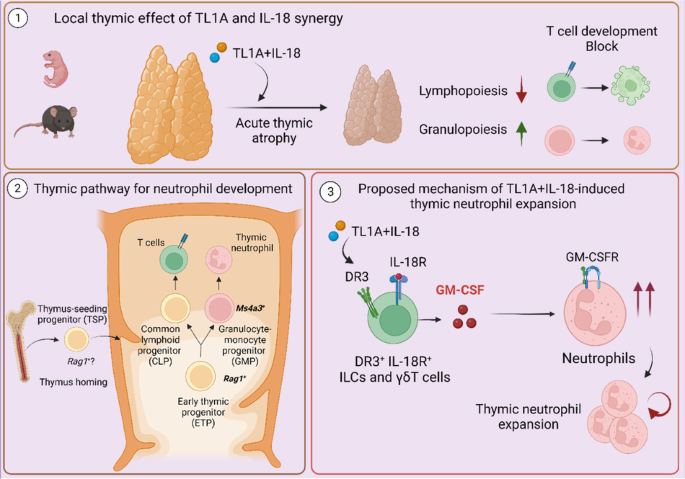
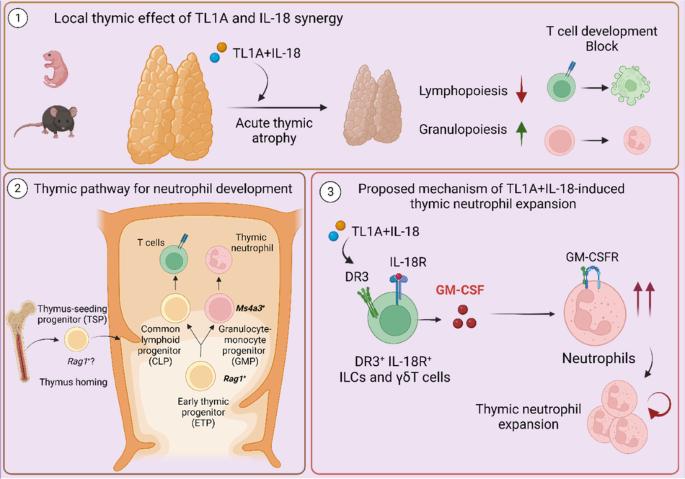
TL1A and IL-18 synergy promotes GM-CSF-dependent thymic granulopoiesis in mice
Acute systemic inflammation critically alters the function of the immune system, often promoting myelopoiesis at the expense of lymphopoiesis. In the thymus, systemic inflammation results in acute thymic atrophy and, consequently, impaired T-lymphopoiesis. The mechanism by which systemic inflammation impacts the thymus beyond suppressing T-cell development is still unclear. Here, we describe how the synergism between TL1A and IL-18 suppresses T-lymphopoiesis to promote thymic myelopoiesis. The protein levels of these two cytokines were elevated in the thymus during viral-induced thymus atrophy infection with murine cytomegalovirus (MCMV) or pneumonia virus of mice (PVM). In vivo administration of TL1A and IL-18 induced acute thymic atrophy, while thymic neutrophils expanded. Fate mapping with Ms4a3-Cre mice demonstrated that thymic neutrophils emerge from thymic granulocyte-monocyte progenitors (GMPs), while Rag1-Cre fate mapping revealed a common developmental path with lymphocytes. These effects could be modeled ex vivo using neonatal thymic organ cultures (NTOCs), where TL1A and IL-18 synergistically enhanced neutrophil production and egress. NOTCH blockade by the LY411575 inhibitor increased the number of neutrophils in the culture, indicating that NOTCH restricted steady-state thymic granulopoiesis. To promote myelopoiesis, TL1A, and IL-18 synergistically increased GM-CSF levels in the NTOC, which was mainly produced by thymic ILC1s. In support, TL1A- and IL-18-induced granulopoiesis was completely prevented in NTOCs derived from Csf2rb-/- mice and by GM-CSFR antibody blockade, revealing that GM-CSF is the essential factor driving thymic granulopoiesis. Taken together, our findings reveal that TL1A and IL-18 synergism induce acute thymus atrophy while promoting extramedullary thymic granulopoiesis in a NOTCH and GM-CSF-controlled manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


