基于 5-硝基异喹啉的 SNH 芳基化反应合成在正位含有亚硝基的二芳基胺的简单方法
IF 1
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在无金属催化剂的条件下,基于氢的氧化亲核取代,一种简单高效的 5-硝基异喹啉芳基化反应得到了证实。该反应可用作合成在正交位置含有亚硝基的异喹啉基二芳基胺的方法,这些化合物具有很高的合成潜力。后者的氧化反应会生成 6-芳基氨基-5-亚硝基异喹啉 N-氧化物。该反应的特点是无需引入离去基团,且总产率高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
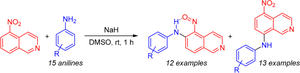
A simple method for the synthesis of diarylamines containing a nitroso group in the ortho position based on the SNH arylamination of 5-nitroisoquinoline
A simple and efficient arylamination reaction of 5-nitroisoquinoline based on the oxidative nucleophilic substitution of hydrogen was demonstrated under metal-catalyst-free conditions. This reaction can be used as a method for the synthesis of isoquinoline-based diarylamines containing a nitroso group in the ortho position, which are compounds with high synthetic potential. The oxidation of the latter leads to the formation of 6-arylamino-5-nitroisoquinoline N-oxides. The absence of the need to introduce leaving groups and a good overall yield are features of this reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
2.90
自引率
13.30%
发文量
98
审稿时长
1 months
期刊介绍:
The international journal Chemistry of Heterocyclic Compounds publishes original papers, short communications, reviews, and mini-reviews dealing with problems in the field of heterocyclic chemistry in Russian and English. The Journal also publishes reviews and annotations on new books and brief reports on conferences in the field of heterocyclic chemistry, as well as commemoratives dedicated to prominent heterocyclic chemists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


