药物动力学和神经免疫药物遗传学对缓释吗啡癌症疼痛控制和不良反应的影响。
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
摘要
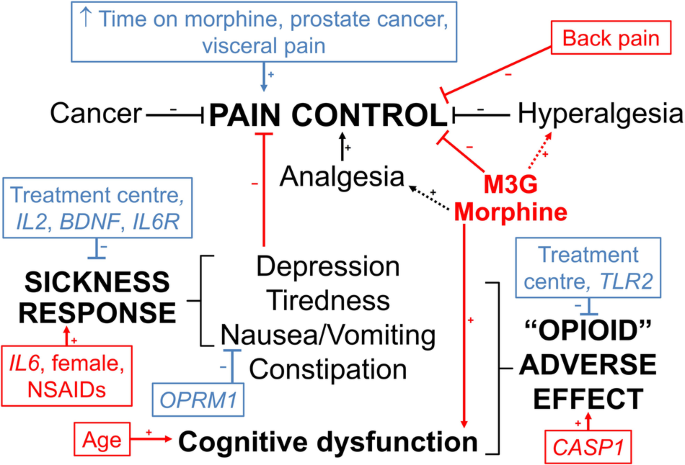
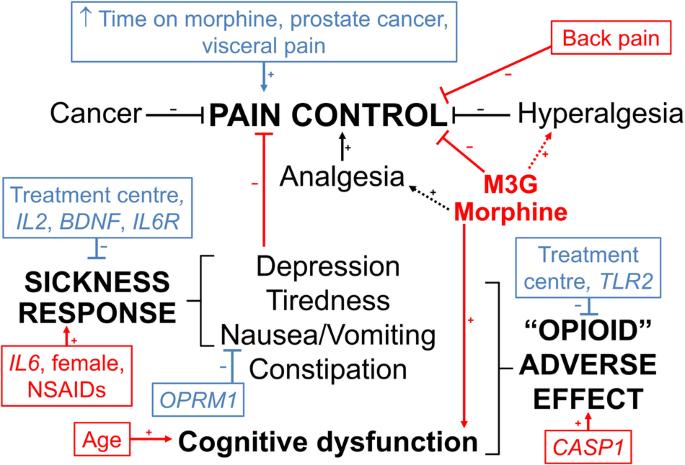
该研究旨在确定接受控释口服吗啡治疗的506名癌症患者的阿片神经免疫药理学通路基因多态性是否会改变血清吗啡、吗啡-3-葡萄糖醛酸和吗啡-6-葡萄糖醛酸的浓度-反应关系。疼痛未得到控制的患者的吗啡-3-葡萄糖醛酸浓度(标准化至给药后 11 小时)较高(中位数(四分位数间距)1.2(0.7-2.3)比 1.0(0.5-1.9)μM,P = 0.006),而认知功能障碍患者的吗啡浓度较高(40(20-81)比 29(14-60)nM,P = 0.02)。TLR2 rs3804100 变异携带者发生阿片类药物不良事件的几率降低(调整后的几率比(95% 置信区间)为 0.42 (0.22-0.82),P = 0.01)。IL2 rs2069762 G/G(0.20 (0.06-0.52))、BDNF rs6265 A/A(0.15 (0.02-0.63))和 IL6R rs8192284 携带者(0.55 (0.34-0.90))基因型的疾病反应几率降低,IL6 rs10499563 C/C增加(3.3 (1.2-9.3))(P ≤ 0.02)。该研究在剂量、取样时间和诊断的异质性方面存在局限性,但仍表明药代动力学和免疫遗传学对癌症患者的吗啡疼痛控制和不良反应有共同作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pharmacokinetic and neuroimmune pharmacogenetic impacts on slow-release morphine cancer pain control and adverse effects
The aim was to determine if opioid neuroimmunopharmacology pathway gene polymorphisms alter serum morphine, morphine-3-glucuronide and morphine-6-glucuronide concentration-response relationships in 506 cancer patients receiving controlled-release oral morphine. Morphine-3-glucuronide concentrations (standardised to 11 h post-dose) were higher in patients without pain control (median (interquartile range) 1.2 (0.7–2.3) versus 1.0 (0.5–1.9) μM, P = 0.006), whereas morphine concentrations were higher in patients with cognitive dysfunction (40 (20–81) versus 29 (14–60) nM, P = 0.02). TLR2 rs3804100 variant carriers had reduced odds (adjusted odds ratio (95% confidence interval) 0.42 (0.22–0.82), P = 0.01) of opioid adverse events. IL2 rs2069762 G/G (0.20 (0.06-0.52)), BDNF rs6265 A/A (0.15 (0.02–0.63)) and IL6R rs8192284 carrier (0.55 (0.34–0.90)) genotypes had decreased, and IL6 rs10499563 C/C increased (3.3 (1.2–9.3)), odds of sickness response (P ≤ 0.02). The study has limitations in heterogeneity in doses, sampling times and diagnoses but still suggests that pharmacokinetics and immune genetics co-contribute to morphine pain control and adverse effects in cancer patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


