吡啶与二酰过氧化物和过氧化氢的副选择性自由基烷基化反应
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报告了一种通过相应的噁嗪基吡啶中间体和廉价的烷基羧酸衍生二酰过氧化物或过氧化物作为烷基自由基前体和内部氧化剂对各种吡啶进行对位选择性烷基化的实用方法。该转化过程无需任何过渡金属催化剂,易于放大,并可用于连续自由基的对位、正位二官能化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
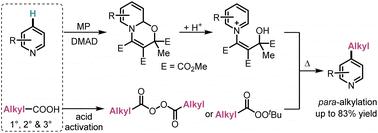
para-Selective radical alkylation of pyridines with diacyl peroxides and peresters†
Herein, a practical method for the para-selective alkylation of various pyridines using the corresponding oxazino pyridine intermediates and cheap alkyl carboxylic acid-derived diacyl peroxides or peresters as alkyl radical precursors and internal oxidants is reported. The transformation proceeds in the absence of any transition metal catalyst, is easy to scale up and can be used in consecutive radical para,ortho-difunctionalization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


