骨巨细胞瘤中的 H3.3-G34W 与外显子选择抑制因子 hnRNPA1L2 在功能上一致
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
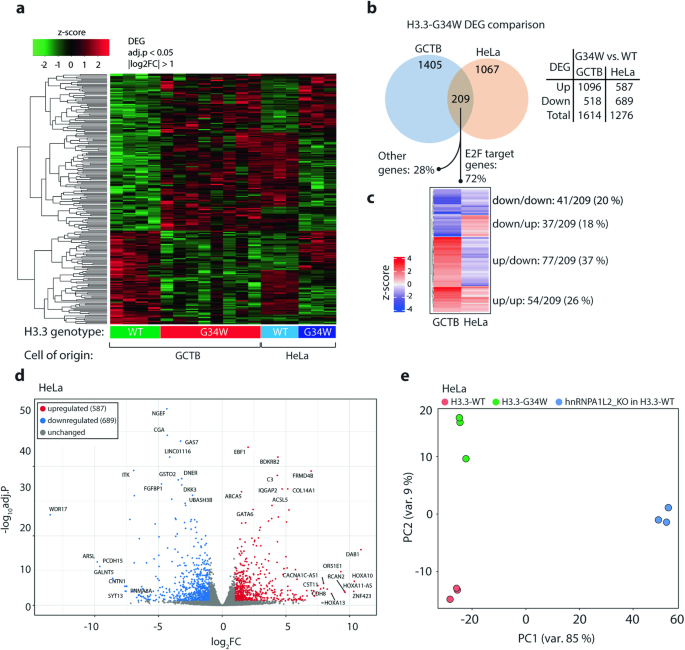
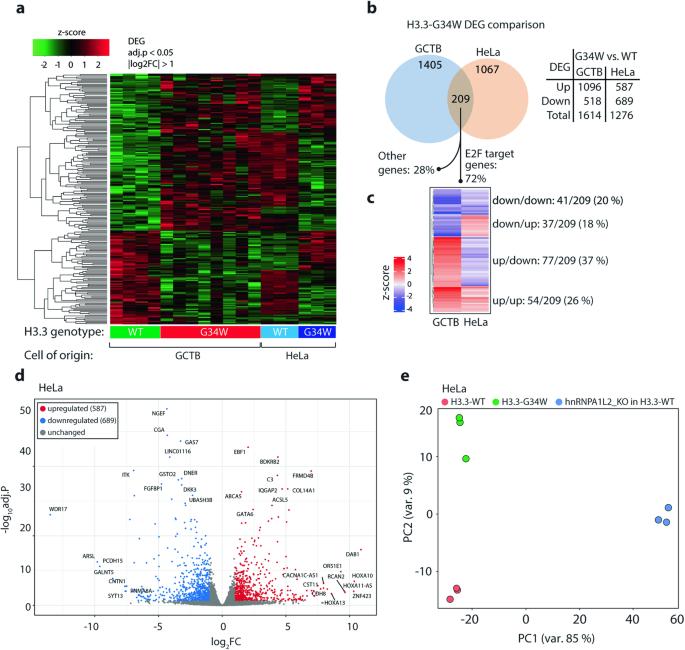
RNA 处理是一种重要的转录后现象,它为翻译前的转录本多样性提供了必要的复杂性。这一过程中的异常可能会导致肿瘤发生,我们以前曾报道过骨巨细胞瘤(GCTB)中剪接改变的增加,这种瘤携带组蛋白变体 H3.3 编码甘氨酸 34 取代色氨酸(H3.3-G34W)的突变。G34W 与多个剪接因子相互作用,其中最显著的是反式作用剪接因子 hnRNPA1L2。为了更深入地了解 GCTB 和带有 H3.3-G34W 的同源 HeLa 细胞中的 RNA 处理过程,我们从 hnRNPA1L2 和 H3.3-G34W 相关 RNA 中生成了 RNA 免疫沉淀测序数据,结果显示 80% 的 RNA 在基因区域中重叠,并经常被注释为 E2F 转录因子结合位点。在含有 H3.3-G34W 的 GCTB 和 HeLa 细胞中,剪接畸变在已知的 hnRNPA1L2 结合基序上明显富集(p 值为 0.01)。这种剪接畸变与 hnRNPA1L2 基因敲除不同,后者显示出独立于 H3.3-G34W 的改变。具有重要功能意义的是,hnRNPA1L2 的重新分布与 H3.3 模式非常吻合,这可能是由 G34W 驱动的,而且是在正常亲本细胞不占据的位点上。综上所述,我们的数据揭示了 hnRNPA1L2 和 H3.3-G34W 之间的功能重叠,这可能会对 GCTB 发病过程中的 RNA 处理产生重大影响。这为今后的治疗干预提供了新的机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
H3.3-G34W in giant cell tumor of bone functionally aligns with the exon choice repressor hnRNPA1L2
RNA processing is an essential post-transcriptional phenomenon that provides the necessary complexity of transcript diversity prior to translation. Aberrations in this process could contribute to tumourigenesis, and we have previously reported increased splicing alterations in giant cell tumor of bone (GCTB), which carries mutations in the histone variant H3.3 encoding glycine 34 substituted for tryptophan (H3.3-G34W). G34W interacts with several splicing factors, most notably the trans-acting splicing factor hnRNPA1L2. To gain a deeper understanding of RNA processing in GCTB and isogenic HeLa cells with H3.3-G34W, we generated RNA-immunoprecipitation sequencing data from hnRNPA1L2 and H3.3-G34W associated RNAs, which showed that 80% overlapped across genic regions and were frequently annotated as E2F transcription factor binding sites. Splicing aberrations in both GCTB and HeLa cells with H3.3-G34W were significantly enriched for known hnRNPA1L2 binding motifs (p value < 0.01). This splicing aberration differed from hnRNPA1L2 knockouts, which showed alterations independent of H3.3-G34W. Of functional significance, hnRNPA1L2 was redistributed to closely match the H3.3 pattern, likely driven by G34W, and to loci not occupied in normal parental cells. Taken together, our data reveal a functional overlap between hnRNPA1L2 and H3.3-G34W with likely significant consequences for RNA processing during GCTB pathogenesis. This provides novel opportunities for therapeutic intervention in future modus operandi.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


