7H-Difurazanofuxanoazepine 和 7H-Trifurasanoazepine 的 N-丙炔基衍生物的热分解动力学
IF 1.4
4区 化学
Q4 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
摘要
摘要 研究了 7H-二呋扎氮杂卓和 7H-三呋扎氮杂卓的丙炔基衍生物在等温和非等温模式下的热稳定性。确定了分解的形式动力学规律和反应速率常数的温度依赖性。比较了氮杂卓的丙炔基、氰甲基、烯丙基和胺衍生物的热稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
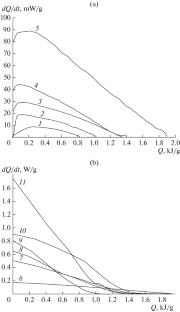
Kinetics of Thermal Decomposition of N-Propargyl Derivatives of 7H-Difurazanofuxanoazepine and 7H-Trifurasanoazepine
The thermal stability of the propargyl derivatives of 7H-difurazanofuroxanoazepine and 7H-trifurazanoazepine in the isothermal and nonisothermal modes is studied. Formal kinetic regularities of the decomposition and temperature dependencies of the reaction rate constants are determined. The thermal stability of propargyl, cyanomethyl, allyl, and amine derivatives of azepines is compared.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Journal of Physical Chemistry B
化学-物理:原子、分子和化学物理
CiteScore
2.20
自引率
71.40%
发文量
106
审稿时长
4-8 weeks
期刊介绍:
Russian Journal of Physical Chemistry B: Focus on Physics is a journal that publishes studies in the following areas: elementary physical and chemical processes; structure of chemical compounds, reactivity, effect of external field and environment on chemical transformations; molecular dynamics and molecular organization; dynamics and kinetics of photoand radiation-induced processes; mechanism of chemical reactions in gas and condensed phases and at interfaces; chain and thermal processes of ignition, combustion and detonation in gases, two-phase and condensed systems; shock waves; new physical methods of examining chemical reactions; and biological processes in chemical physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


