CircPKN2通过促进硬脂酰-CoA去饱和酶1的泛素化来促进膀胱癌中的铁变态反应。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
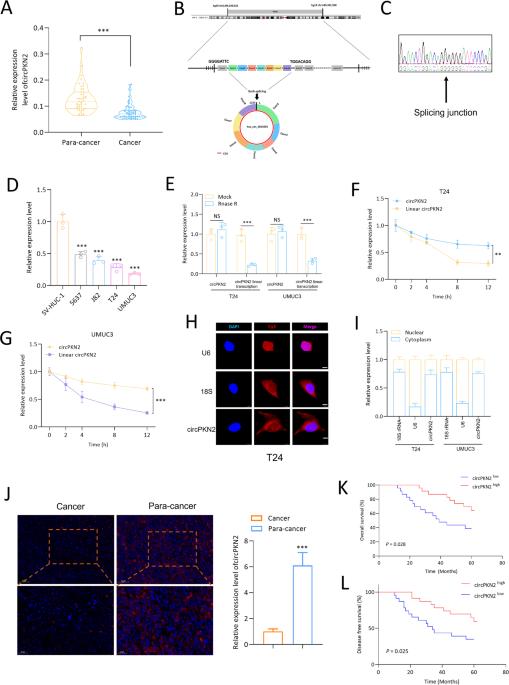
膀胱癌(BC)是男性泌尿系统最常见的恶性肿瘤之一,目前缺乏最佳治疗策略。为了从循环 RNA 的角度阐明膀胱癌的致病机制,我们开展了这项研究。在以往研究的基础上,我们对一种新型循环 RNA circPKN2 产生了浓厚的兴趣,因为它在 BC 中显著下调,而且与 BC 患者的预后密切相关。我们的研究结果表明,circPKN2 可以抑制 BC 细胞在体外的增殖和迁移。此外,我们还发现 circPKN2 通过促进铁凋亡对 BC 发挥抗癌作用。机理研究发现,circPKN2可招募STUB1,促进SCD1泛素化,从而抑制WNT通路,促进铁凋亡。此外,我们的研究还揭示了剪接因子QKI在circPKN2生物发生过程中的调控作用。动物实验证明,circPKN2 能增强 BC 细胞体内的铁凋亡,抑制肿瘤生长和转移。抗癌因子 circPKN2 的发现有望为预防和治疗 BC 提供新的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CircPKN2 promotes ferroptosis in bladder cancer by promoting the ubiquitination of Stearoyl-CoA Desaturase 1
Bladder cancer (BC) is one of the most common malignancies in the male urinary system and currently lacks an optimal treatment strategy. To elucidate the pathogenic mechanisms of BC from the perspective of circular RNAs, we conducted this study. Building upon our previous research, a novel circRNA, circPKN2, captured our interest due to its significant downregulation in BC, and its close association with the prognosis of BC patients. Our research findings indicate that circPKN2 can inhibit the proliferation and migration of BC cells in vitro. Furthermore, we discovered that circPKN2 exerts its anti-cancer effects in BC by promoting ferroptosis. Mechanistic studies revealed that circPKN2 recruits STUB1 to facilitate the ubiquitination of SCD1, thereby suppressing the WNT pathway and promoting ferroptosis in BC. Additionally, our research unveiled the regulatory role of the splicing factor QKI in the biogenesis of circPKN2. Animal studies demonstrated that circPKN2 enhances ferroptosis in BC cells in vivo, inhibiting tumor growth and metastasis. The discovery of the anti-cancer factor circPKN2 holds promise for providing new therapeutic targets in the prevention and treatment of BC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


