在哈钦森-吉尔福特早衰综合征中,细胞质 EP300 对 mTORC1 的致病性过度激活。
IF 3
Q2 CELL BIOLOGY
引用次数: 0
摘要
在最近一期《自然-细胞生物学》(Nature Cell Biology)杂志上,Sung Min Son 等人揭示了 EP300 在调控 mTORC1/自噬轴过程中的一个新层次。该研究强调,在哈钦森-吉尔福德早衰综合征中,过量的细胞质 EP300 会导致 mTORC1 过度激活和自噬功能受损,从而可能导致早衰和加速衰老。本文章由计算机程序翻译,如有差异,请以英文原文为准。
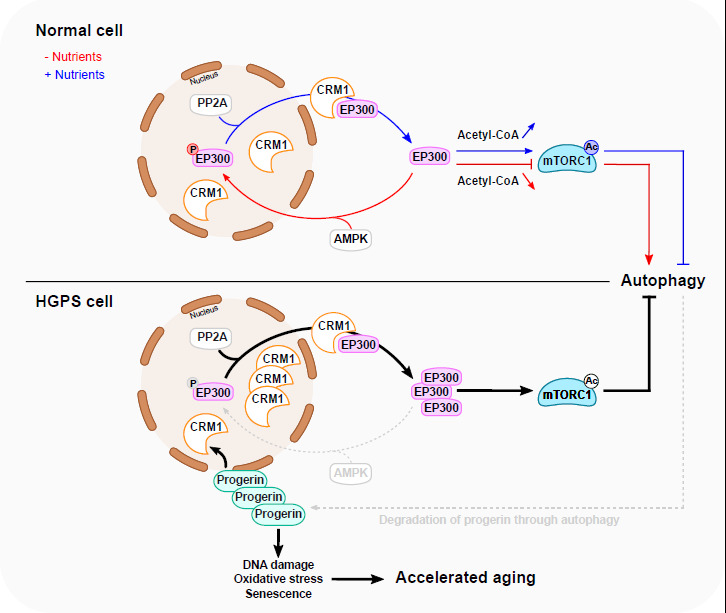
Pathogenic hyperactivation of mTORC1 by cytoplasmic EP300 in Hutchinson-Gilford progeria syndrome.
In a recent issue in Nature Cell Biology, Sung Min Son et al. unveil a novel layer in the regulation of the mTORC1/autophagy axis by EP300 which can undergo nucleocytoplasmic shuttling in response to alterations in nutrient availability. The study highlights that, in Hutchinson-Gilford progeria syndrome, overabundant cytoplasmic EP300 results in mTORC1 hyperactivation and impaired autophagy, potentially contributing to premature and accelerated aging.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Stress
Biochemistry, Genetics and Molecular Biology-Biochemistry, Genetics and Molecular Biology (miscellaneous)
CiteScore
13.50
自引率
0.00%
发文量
21
审稿时长
15 weeks
期刊介绍:
Cell Stress is an open-access, peer-reviewed journal that is dedicated to publishing highly relevant research in the field of cellular pathology. The journal focuses on advancing our understanding of the molecular, mechanistic, phenotypic, and other critical aspects that underpin cellular dysfunction and disease. It specifically aims to foster cell biology research that is applicable to a range of significant human diseases, including neurodegenerative disorders, myopathies, mitochondriopathies, infectious diseases, cancer, and pathological aging.
The scope of Cell Stress is broad, welcoming submissions that represent a spectrum of research from fundamental to translational and clinical studies. The journal is a valuable resource for scientists, educators, and policymakers worldwide, as well as for any individual with an interest in cellular pathology. It serves as a platform for the dissemination of research findings that are instrumental in the investigation, classification, diagnosis, and therapeutic management of major diseases. By being open-access, Cell Stress ensures that its content is freely available to a global audience, thereby promoting international scientific collaboration and accelerating the exchange of knowledge within the research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


