(±)-恩替卡韦的合成
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
摘要 在科里(±)-内酯二元醇的基础上开发出了(±)-恩替卡韦的实用合成方法。其关键步骤是通过氧化脱羧 2-{(1S*、2-{(1S*,2R*,3S*,5R*)-5-{[叔丁基(二甲基)硅烷基]氧基}-3-(1-乙氧基乙氧基)-2-[(1-乙氧基乙氧基)甲基]环戊基}乙酸与四乙酸铅的氧化脱羧反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
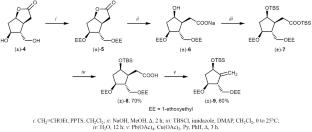
Synthesis of (±)-Entecavir
A practical synthesis of (±)-entecavir has been developed on the basis of Corey (±)-lactone diol. The key stage is the synthesis of (1R*,3R*,4S*)-4-(1-ethoxyethoxy)-3-[(1-ethoxyethoxy)methyl]-2-methylidenecyclopentan-1-ol by oxidative decarboxylation of 2-{(1S*,2R*,3S*,5R*)-5-{[tert-butyl(dimethyl)silyl]oxy}-3-(1-ethoxyethoxy)-2-[(1-ethoxyethoxy)methyl]cyclopentyl}acetic acid with lead tetraacetate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


