基于生物活性 (5Z,9Z)-二十二碳-5,9-二烯酸的酰胺的合成
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
摘要 通过Cp2TiCl2催化脂肪族和含氧1,2-二烯的分子间交叉环镁化反应作为关键步骤,合成了(5Z,9Z)-二十二碳-5,9-二烯酸的新型酰胺,它是一种高活性拓扑异构酶I/II抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
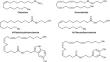
Synthesis of Amides Based on Biologically Active (5Z,9Z)-Eicosa-5,9-dienoic Acid
Abstract
New amides of (5Z,9Z)-eicosa-5,9-dienoic acid, which is a highly active topoisomerase I/II inhibitor, were synthesized via Cp2TiCl2-catalyzed intermolecular cross-cyclomagnesiation of aliphatic and oxygen-containing 1,2-dienes as the key step.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


