中性粒细胞通过靶向 Siglec-G 破坏 B-1a 细胞稳态,从而加剧败血症。
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
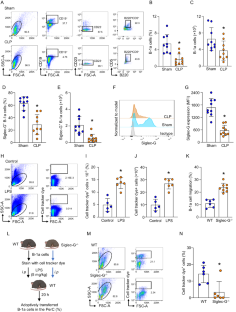
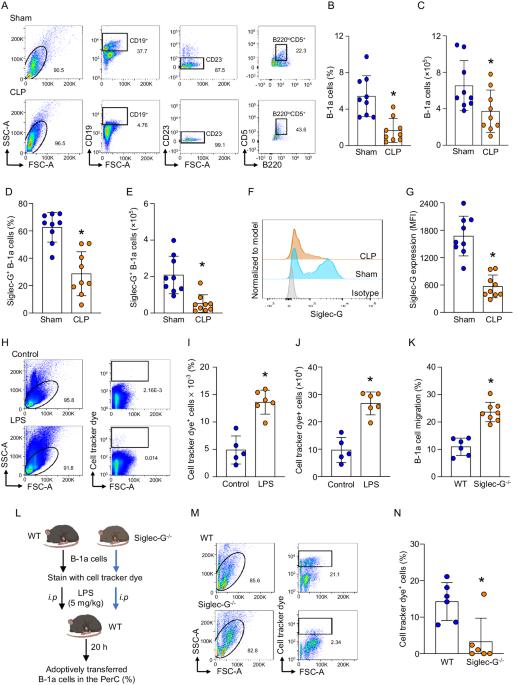
B-1a 细胞是一种先天性类细胞群,通过释放天然 IgM 和 IL-10 对病原体防御和炎症调节至关重要。在败血症时,腹腔中的 B-1a 细胞数量会减少,因为它们会大量迁移到脾脏。在脾脏内,迁移的 B-1a 细胞分化成浆细胞,导致其原有的表型和功能发生改变。我们发现了一种关键的作用因子--唾液酸结合免疫球蛋白样凝集素-G(Siglec-G),它主要在B-1a细胞上表达,负向调节B-1a细胞的迁移以维持体内平衡。Siglec-G 与 CXCR4/CXCL12 相互作用,调节 B-1a 细胞的迁移。中性粒细胞通过中性粒细胞弹性蛋白酶(NE)介导的 Siglec-G 裂解来帮助 B-1a 细胞迁移。人体研究显示,脓毒症患者体内 NE 表达增加。我们在硅学中确定了 NE 的裂解序列,从而发现了一种诱饵肽,它能保护 Siglec-G、保护腹膜 B-1a 细胞、减轻炎症反应并提高脓毒症患者的存活率。Siglec-G在抑制B-1a细胞迁移以维持其固有表型和功能方面的作用在脓毒症中会受到NE的损害,这为了解B-1a细胞的稳态提供了宝贵的信息。采用一种小型诱饵肽来阻止 NE 介导的 Siglec-G 裂解,已成为一种有希望维持腹膜 B-1a 细胞稳态、缓解炎症并最终改善脓毒症患者预后的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Neutrophils disrupt B-1a cell homeostasis by targeting Siglec-G to exacerbate sepsis
B-1a cells, an innate-like cell population, are crucial for pathogen defense and the regulation of inflammation through their release of natural IgM and IL-10. In sepsis, B-1a cell numbers are decreased in the peritoneal cavity as they robustly migrate to the spleen. Within the spleen, migrating B-1a cells differentiate into plasma cells, leading to alterations in their original phenotype and functionality. We discovered a key player, sialic acid-binding immunoglobulin-like lectin-G (Siglec-G), which is expressed predominantly on B-1a cells and negatively regulates B-1a cell migration to maintain homeostasis. Siglec-G interacts with CXCR4/CXCL12 to modulate B-1a cell migration. Neutrophils aid B-1a cell migration via neutrophil elastase (NE)-mediated Siglec-G cleavage. Human studies revealed increased NE expression in septic patients. We identified an NE cleavage sequence in silico, leading to the discovery of a decoy peptide that protects Siglec-G, preserves peritoneal B-1a cells, reduces inflammation, and enhances sepsis survival. The role of Siglec-G in inhibiting B-1a cell migration to maintain their inherent phenotype and function is compromised by NE in sepsis, offering valuable insights into B-1a cell homeostasis. Employing a small decoy peptide to prevent NE-mediated Siglec-G cleavage has emerged as a promising strategy to sustain peritoneal B-1a cell homeostasis, alleviate inflammation, and ultimately improve outcomes in sepsis patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


