tRF-33-P4R8YP9LON4VDP 通过调节 STAT3 信号通路以 AGO2 依赖性方式抑制胃癌进展。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
已有研究表明,tRNA 衍生的小 RNA(tsRNA)在癌症的病理生理学中发挥着重要功能。在本研究中,我们重点研究了 tRF-33-P4R8YP9LON4VDP(tRF-33)在胃恶性肿瘤发生发展中的可能机制。我们共收集了 454 份不同胃黏膜病变的组织样本。测定了不同组群中 tRF-33 的表达水平,并评估了其诊断效率和预后评估价值。采用细胞增殖试验、Transwell 试验、流式细胞术和异种移植模型来评估其对胃癌细胞的作用。荧光原位杂交、双荧光素酶检测、Western 印迹和 RNA 结合蛋白免疫沉淀验证了其分子机制。结果表明,从正常对照样本到胃炎组织、早期和潜伏期胃癌组织,tRF-33 的表达均呈逐渐变化的趋势。因此,tRF-33 作为胃恶性肿瘤的预测和诊断生物标记物具有巨大潜力。过度表达 tRF-33 可抑制胃癌细胞的进展和转移活力,并诱导细胞凋亡。裸鼠的致瘤性显示了 tRF-33 的抑制特性。机理研究发现,tRF-33 通过与 AGO2 结合对 STAT3 mRNA 发挥抑制作用。总之,tRF-33 具有诊断胃癌和评估其预后的价值,并能通过抑制 STAT3 信号通路抑制肿瘤细胞的活力。tRF-33 与 AGO2 蛋白结合后,通过靶向 STAT3 的 3'UTR 负向调节 STAT3 的表达。STAT3 的表达下调导致 STAT3 和 p-STAT3 的减少,并进一步阻断下游基因的转录,最终抑制胃癌的发生。MMP-9,基质金属蛋白酶-9;Bcl-2,B细胞淋巴瘤-2;STAT3,信号转导和激活转录3;UTR,非翻译区。本文章由计算机程序翻译,如有差异,请以英文原文为准。
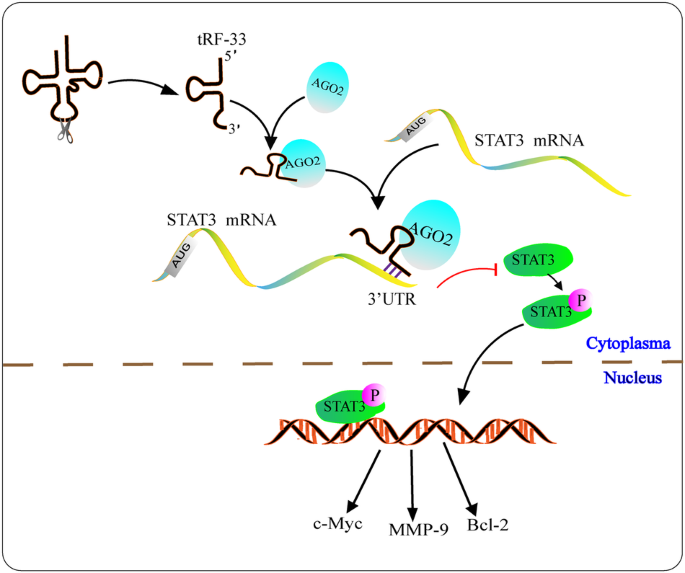

tRF-33-P4R8YP9LON4VDP inhibits gastric cancer progression via modulating STAT3 signaling pathway in an AGO2-dependent manner
It has been demonstrated that tRNA-derived small RNAs (tsRNAs) perform essential functions in the pathophysiology of cancer. In this study, we focused on the possible mechanisms of tRF-33-P4R8YP9LON4VDP (tRF-33) underlying the development of gastric malignancy. In total, 454 tissue samples with different gastric mucosal lesions were collected. The tRF-33 expression level in different cohorts was determined, and its value for diagnostic efficiency and prognosis evaluation were assessed. Cell proliferation assays, Transwell assay, flow cytometry, and xenotransplantation model were used to evaluate its effect on gastric cancer cells. The molecular mechanism was verified by fluorescence in situ hybridization, dual luciferase assay, Western blot, and RNA binding protein immunoprecipitation. The results showed that the expression of tRF-33 exhibited a gradual modification from normal control samples to gastritis tissues, early and latent stage of gastric cancer tissues. Consequently, tRF-33 holds significant potential as a predictive and diagnostic biomarker for gastric malignancy. Over-expression of tRF-33 inhibited gastric cancer cell progression and metastatic viability, and induced cell apoptosis. Tumorigenicity in nude mice showed the suppressive characteristics of tRF-33. Mechanistic investigation revealed that tRF-33 exerted silencing on STAT3 mRNA via binding to AGO2. In conclusion, tRF-33 exhibited values in diagnosing gastric cancer and evaluating its prognosis, and suppressed tumor cell viability by inhibiting STAT3 signaling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


