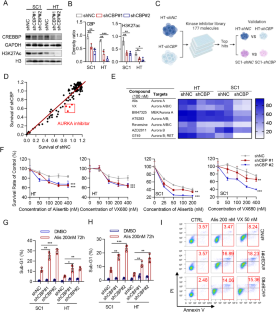
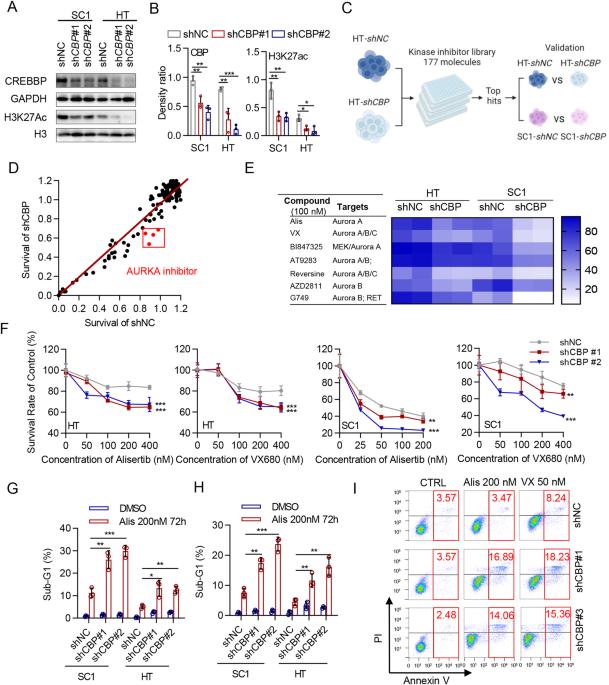
以 AURKA 为靶点,通过抑制 MYC 的表达,诱导 CREBBP 缺失型 B 细胞恶性肿瘤的合成致死率。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
CREBBP 是一种组蛋白乙酰转移酶的编码,其功能缺失突变经常发生在 B 细胞恶性肿瘤中,这突出表明 CREBBP 缺乏是一个有吸引力的治疗靶点。利用已建立的同源细胞模型,我们证明了CREBBP缺陷细胞对AURKA抑制的选择性易感性。从机理上讲,我们发现联合靶向 CREBBP 和 AURKA 可抑制 MYC 的转录和翻译后作用,从而诱导复制压力和细胞凋亡。抑制AURKA可显著降低CREBBP缺陷细胞中的MYC蛋白水平,这意味着维持MYC稳定性需要依赖AURKA。此外,体内研究表明,药理抑制AURKA可有效延缓CREBBP缺陷细胞的肿瘤进展,并与CREBBP抑制剂协同作用于CREBBP缺陷细胞。我们的研究揭示了CREBBP和AURKA之间新的合成致死相互作用,表明针对AURKA是治疗携带CREBBP失活突变的高危B细胞恶性肿瘤的一种潜在治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting AURKA to induce synthetic lethality in CREBBP-deficient B-cell malignancies via attenuation of MYC expression
Loss-of-function mutations in CREBBP, which encodes for a histone acetyltransferase, occur frequently in B-cell malignancies, highlighting CREBBP deficiency as an attractive therapeutic target. Using established isogenic cell models, we demonstrated that CREBBP-deficient cells are selectively vulnerable to AURKA inhibition. Mechanistically, we found that co-targeting CREBBP and AURKA suppressed MYC transcriptionally and post-translationally to induce replication stress and apoptosis. Inhibition of AURKA dramatically decreased MYC protein level in CREBBP-deficient cells, implying a dependency on AURKA to sustain MYC stability. Furthermore, in vivo studies showed that pharmacological inhibition of AURKA was efficacious in delaying tumor progression in CREBBP-deficient cells and was synergistic with CREBBP inhibitors in CREBBP-proficient cells. Our study sheds light on a novel synthetic lethal interaction between CREBBP and AURKA, indicating that targeting AURKA represents a potential therapeutic strategy for high-risk B-cell malignancies harboring CREBBP inactivating mutations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


