镍催化芳基卤化物的逆选择性跨亲电偶合:通向多种 MOP 型配体的通用实用途径。
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们报告了正(氯)芳基膦氧化物与正(溴)芳基醚之间的高度交叉和反向选择性偶联。这种以前未知的不对称镍催化反应为获得其他方法难以获得的高对映体轴向手性双芳基单膦氧化物提供了一条直接途径。这些产物很容易被还原生成具有复杂骨架的手性 MOP 型配体。这些手性配体在不对称催化中的用途也得到了证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
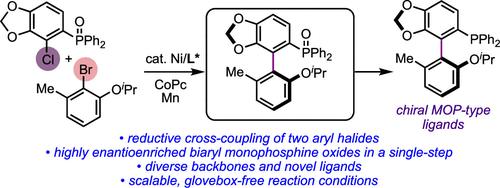
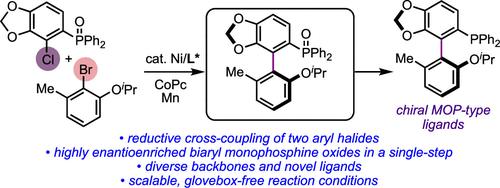
Nickel-Catalyzed Atroposelective Cross-Electrophile Coupling of Aryl Halides: A General and Practical Route to Diverse MOP-Type Ligands
We report a highly cross- and atroposelective coupling between ortho-(chloro)arylphosphine oxides and ortho-(bromo)aryl ethers. This previously unknown asymmetric nickel-catalyzed reaction offers a direct route to highly enantioenriched axially chiral biaryl monophosphine oxides that are difficult to access by other means. These products can be readily reduced to generate chiral MOP-type ligands bearing complex skeletal backbones. The utility of these chiral ligands in asymmetric catalysis is also demonstrated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


