使用烷基咔嗪酸盐无金属合成 C-3-烷氧基羰基 2H-Indazoles
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在室温无金属条件下,利用烷基咔嗪酸盐直接对 2H-indazoles 进行 C-3-alkoxycarbonylation 的简单、高效和环保方法已经开发出来。目前的这一方法可以方便地获得 C-3-羧酸酯衍生的 2H-吲唑,且具有广泛的官能团耐受性,收率良好甚至极佳。机理研究表明,反应是通过自由基途径进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
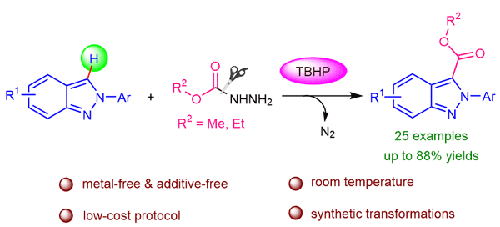
Metal-Free Synthesis of C-3-Alkoxycarbonylated 2H-Indazoles Using Alkyl Carbazates
A simple, efficient, and environmentally benign method for the direct C-3-alkoxycarbonylation of 2H-indazoles using alkyl carbazates has been developed under metal-free conditions at room temperature. This current protocol represents a facile access to C-3-carboxylic ester derived 2H-indazoles with wide functional group tolerance in good to excellent yields. The mechanistic studies suggest that the reaction proceeds through a radical pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


