通过靶向 MANF-HSF1-HSP70-1 通路重编程肿瘤相关巨噬细胞并抑制肿瘤新生血管:肝细胞癌的有效治疗方法
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
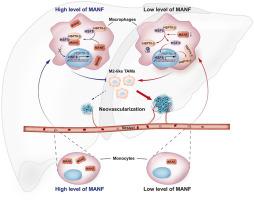
在晚期肝细胞癌(HCC)组织中,M2 样肿瘤相关巨噬细胞(TAMs)占大多数,并促进 HCC 的进展。与 M2 样肿瘤相关巨噬细胞的促癌作用相反,M1 样肿瘤相关巨噬细胞占一小部分,具有抗肿瘤作用。由于 TAMs 可以从一种类型转换到另一种类型,因此对 TAMs 进行重编程可能是治疗 HCC 的一种重要方法。然而,表型转换和重编程 TAMs 的机制仍不清楚。在这项研究中,我们分析了正常巨噬细胞和TAMs中的差异基因,发现TAMs中MANF的缺失伴随着高水平的受MANF负调控的下游基因。MANF 使 TAMs 重编程为 M1 表型。同时,MANF的缺失促进了HCC患者和小鼠HCC模型中HCC的进展,尤其是肿瘤新生血管的形成。此外,巨噬细胞补充MANF可抑制小鼠的HCC进展,表明巨噬细胞补充MANF可有效治疗HCC。从机理上讲,MANF增强了HSF1-HSP70-1的相互作用,限制了巨噬细胞胞质中HSF1的活性,降低了HSP70-1的mRNA和蛋白水平,从而导致TAMs重编程,抑制了HCC的新生血管形成。我们的研究有助于探索TAMs重编程的机制,这可能为未来治疗利用HCC新生血管提供启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Reprogramming tumor-associated macrophages and inhibiting tumor neovascularization by targeting MANF–HSF1–HSP70-1 pathway: An effective treatment for hepatocellular carcinoma
In advanced hepatocellular carcinoma (HCC) tissues, M2-like tumor-associated macrophages (TAMs) are in the majority and promotes HCC progression. Contrary to the pro-tumor effect of M2-like TAMs, M1-like TAMs account for a small proportion and have anti-tumor effects. Since TAMs can switch from one type to another, reprogramming TAMs may be an important treatment for HCC therapy. However, the mechanisms of phenotypic switch and reprogramming TAMs are still obscure. In this study, we analyzed differential genes in normal macrophages and TAMs, and found that loss of MANF in TAMs accompanied by high levels of downstream genes negatively regulated by MANF. MANF reprogrammed TAMs into M1 phenotype. Meanwhile, loss of MANF promoted HCC progression in HCC patients and mice HCC model, especially tumor neovascularization. Additionally, macrophages with MANF supplement suppressed HCC progression in mice, suggesting MANF supplement in macrophage was an effective treatment for HCC. Mechanistically, MANF enhanced the HSF1–HSP70-1 interaction, restricted HSF1 in the cytoplasm of macrophages, and decreased both mRNA and protein levels of HSP70-1, which in turn led to reprogramming TAMs, and suppressing neovascularization of HCC. Our study contributes to the exploration the mechanism of TAMs reprogramming, which may provide insights for future therapeutic exploitation of HCC neovascularization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


