在无催化剂和无溶剂条件下单锅和三组分偶联合成新型对位((苯并噻唑基氨基)(芳基/杂芳基)甲基)苯酚及其相应的 O-对甲苯磺酸盐
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过百里酚或香芹酚、芳基/杂芳基醛和 2-氨基苯并噻唑的单锅三组分偶联反应,开发了一种无催化剂和溶剂的对位((苯并噻唑基氨基)(芳基/杂芳基)甲基)官能化苯酚及其 O-对甲苯磺酸盐的合成方法,并具有很高的选择性。目前的氨基甲基化工艺即使大规模实施也很方便,范围广泛。这些产物很可能是通过百里酚对醛的初始对位反应生成对位苯醌甲酰肼中间体,然后 2-氨基苯并噻唑对原位生成的对位苯醌甲酰肼中间体进行 1,6-Aza-Michael 加成反应而形成的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
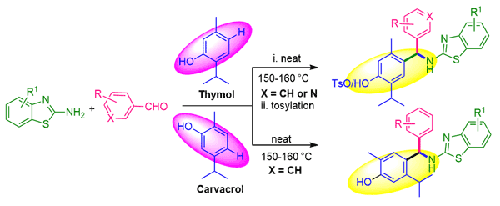
One-pot and Three Component Coupling Synthesis of Novel para-((Benzothiazolylamino)(aryl/heteroaryl)methyl)-phenols and its Corresponding O-Tosylates under Catalyst- and Solvent-Free Conditions
A catalyst- and solvent-free procedure has been developed for the synthesis of para-(( Benzothiazolylamino)(aryl/heteroaryl)methyl)-functionalized phenols and its O-tosylates via one-pot three component coupling reaction of thymol or carvacrol, aryl/heteroaryl aldehydes, and 2-aminobenzothiazoles with high selectivity. The present amino methylation process is convenient to perform even on large scale with a broad scope. The products were likely formed through the initial para-attack of thymol on aldehydes to generate para-quinone methide intermediate and subsequent 1,6-Aza-Michael addition of 2-aminobenzothiazoles on in-situ generated para-quinone methide intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


