手性胍催化的介-茚-1,3-二醇的对映选择性硅烷化不对称反应
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
使用氯硅烷通过对映选择性硅烷化催化实现了介-茚-1,3-二醇的手性胍基不对称反应,产率高,选择性强。氯硅烷和催化剂的组合取决于底物 C-2 位上的取代基。研究发现,底物上的融合苯基环对实现高选择性至关重要。在优化的反应条件下,发现所提出的方法适用于多种类型的底物。通过观察发现,双硅烷化动力学解析与添加剂 Horeau 放大可实现高选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
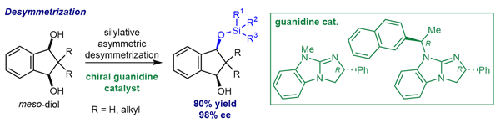
Enantioselective Silylative Desymmetrization of meso-Indane-1,3-diols Catalyzed by Chiral Guanidines
Chiral guanidine-catalyzed desymmetrization of meso-indan-1,3-diols was achieved via enantioselective silylation by using chlorosilanes in good yields with high selectivity. The combination of chlorosilanes and catalysts was determined by the substituents at the C-2 position on the substrate. It was found that the fused phenyl ring on the substrate was essential for achieving high selectivity. The proposed method was found to be applicable to several types of substrates under optimized reaction conditions. Double silylative kinetic resolution with additive Horeau amplification was observed to establish high selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


