乌基酸后催化破碎和捕获:实现新型双(吲哚基)乙酰胺的前所未有的方法
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究人员开发了一种乌基后布氏酸催化的前所未有的碎裂方法,然后通过吲哚亲核剂对亚烷基吲哚啉中间体进行原位捕获,从而生成新型的双(吲哚基)乙酰胺。在这种酸催化的 Ugi 加合物破碎过程中形成的酰胺片段也被分离出来并进行了表征。巧妙地选择了 Ugi 反应中的羧酸和胺成分,以便通过简单的水洗去除酰胺片段,从而获得所需的纯双(吲哚基)乙酰胺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
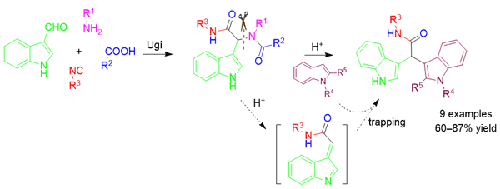
Post-Ugi acid catalyzed fragmentation and trapping: An unprecedented approach towards novel bis(indolyl)acetamides
A Post-Ugi Brønsted acid catalyzed unprecedented fragmentation followed by in situ trapping of the alkylideneindolenine intermediate by indole nucleophiles is developed to furnish novel bis(indolyl)acetamides. The amide fragment formed during this acid catalyzed fragmentation of Ugi adduct was also isolated and characterized. The carboxylic acid and amine components of Ugi reaction were smartly chosen to allow simple water wash for removal of amide fragment to obtain the desired bis(indolyl)acetamides in pure form.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


