通过重氮甲烷介导的 α-ketoN,O-hemiaminals 的 CC 键裂解合成环戊烷环化 δ 内酰胺
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
首次研究了重氮甲烷介导的α-酮 N,O-半氨分子的 C-C 键裂解。重氮甲烷作为试剂导致降冰片烷基α-酮 N,O-半酰胺的 C-C 键裂解,产生类似 Grob 的碎片,从而形成环戊烷环化的δ-内酰胺,产量极高。我们利用各种胺对降冰片α-二酮的卤素进行亲核置换,合成了前体降冰片α-酮 N,O-半酰胺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
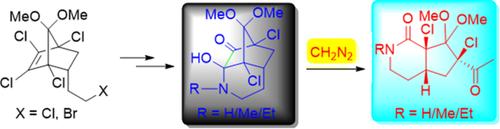
Synthesis of cyclopentane annulated δ-lactams via diazomethane-mediated CC bond cleavage of α-keto N,O-hemiaminals
Diazomethane-mediated C-C bond cleavage of α-keto N,O-hemiaminals was investigated for the first time. Diazomethane as a reagent result C-C bond cleavage of norbornyl α-keto N,O-hemiaminals, Grob-like fragmentation, lead to the formation of cyclopentane annulated δ-lactams in excellent yields. We have synthesized the precursor norbornyl α-keto N,O-hemiaminal by the nucleophilic displacement of halogen of norbornyl α-diketone with the various amines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


