中性粒细胞-巨噬细胞通过细胞外囊泡转移进行交流,促进了伊塔康酸的积累并改善了细胞因子风暴综合征。
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
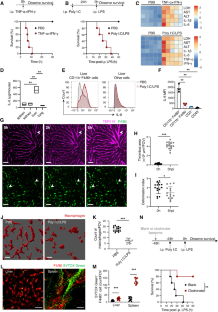
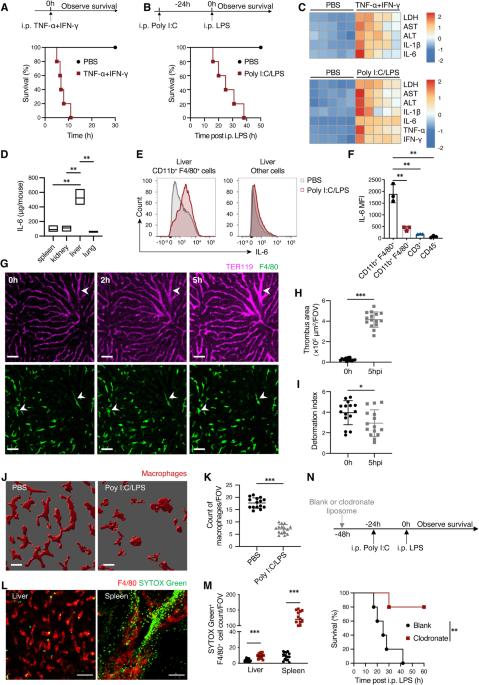
细胞因子风暴综合征(CSS)是一种危及生命的全身性炎症综合征,涉及由各种疗法、感染和自身免疫疾病引发的先天性免疫亢进。然而,先天性免疫细胞之间潜在的相互作用尚未完全明了。本文利用多聚 I:C 和脂多糖(LPS)诱导的细胞因子风暴模型,确定了中性粒细胞在 CSS 模型中通过调节巨噬细胞活化起到的保护作用。肉眼成像显示肝脏和脾脏中有中性粒细胞衍生的细胞外囊泡 (NDEV),这些囊泡被巨噬细胞捕获。NDEVs在体外共培养或注入CSS模型时可抑制巨噬细胞产生促炎细胞因子。经 NDEV 处理的巨噬细胞的代谢图谱显示,抗炎代谢物伊他康酸的水平升高,伊他康酸是由顺式乌头酸脱羧酶(Acod1,由 Irg1 编码)在克雷布斯循环中从顺式乌头酸产生的。巨噬细胞(而非中性粒细胞)中的 Irg1 对 NDEV 介导的抗炎作用至关重要。从机制上讲,NDEV 传递 miR-27a-3p,抑制了 Suclg1 的表达,而 Suclg1 是伊他康酸代谢酶的编码基因,因此导致了伊他康酸在巨噬细胞中的积累。这些研究结果表明,由细胞外囊泡介导的中性粒细胞与巨噬细胞之间的通讯对于促进 CSS 中巨噬细胞的抗炎重编程至关重要,可能对治疗这种致命疾病具有潜在的意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Neutrophil–macrophage communication via extracellular vesicle transfer promotes itaconate accumulation and ameliorates cytokine storm syndrome
Cytokine storm syndrome (CSS) is a life-threatening systemic inflammatory syndrome involving innate immune hyperactivity triggered by various therapies, infections, and autoimmune conditions. However, the potential interplay between innate immune cells is not fully understood. Here, using poly I:C and lipopolysaccharide (LPS)-induced cytokine storm models, a protective role of neutrophils through the modulation of macrophage activation was identified in a CSS model. Intravital imaging revealed neutrophil-derived extracellular vesicles (NDEVs) in the liver and spleen, which were captured by macrophages. NDEVs suppressed proinflammatory cytokine production by macrophages when cocultured in vitro or infused into CSS models. Metabolic profiling of macrophages treated with NDEV revealed elevated levels of the anti-inflammatory metabolite, itaconate, which is produced from cis-aconitate in the Krebs cycle by cis-aconitate decarboxylase (Acod1, encoded by Irg1). Irg1 in macrophages, but not in neutrophils, was critical for the NDEV-mediated anti-inflammatory effects. Mechanistically, NDEVs delivered miR-27a-3p, which suppressed the expression of Suclg1, the gene encoding the enzyme that metabolizes itaconate, thereby resulting in the accumulation of itaconate in macrophages. These findings demonstrated that neutrophil-to-macrophage communication mediated by extracellular vesicles is critical for promoting the anti-inflammatory reprogramming of macrophages in CSS and may have potential implications for the treatment of this fatal condition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


