GITR通过对NLRP3的翻译后调控,加剧脓毒症中溶血磷脂酰胆碱诱导的巨噬细胞脓毒症。
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
NLRP3 炎性体是炎症的驱动因子,但它与早期脓毒症脂质代谢变化的关系仍不清楚。在这里,我们发现溶血磷脂酰胆碱(LPC)会诱导单核细胞/巨噬细胞中 GITR 的表达,并且与脓毒症的严重程度呈正相关。GITR 是一种成本刺激分子,主要在 T 细胞中表达,但其在巨噬细胞中的功能尚不清楚。我们的体外研究数据显示,GITR 可增强巨噬细胞对 LPC 的吸收,并特异性地增强 NLRP3 炎症体介导的巨噬细胞脓毒症。此外,使用盲肠结扎和穿刺(CLP)或 LPS 诱导的脓毒症模型进行的体内研究表明,LPC 会加重脓毒症的严重程度/致死率,而在骨髓细胞中条件性敲除 GITR 或 NLRP3/caspase-1/IL-1β 缺乏会减轻脓毒症的严重程度/致死率。从机制上讲,GITR通过调节NLRP3的翻译后修饰(PTM)特异性地增强了炎性体的激活。GITR与NLRP3竞争结合到E3连接酶MARCH7上,并招募MARCH7诱导去乙酰化酶SIRT2降解,导致NLRP3的泛素化减少而乙酰化增加。总之,这些发现揭示了巨噬细胞源性 GITR 在调节 NLRP3 的 PTM 和全身炎症损伤中的新作用,表明 GITR 可能是败血症和其他炎症性疾病的潜在治疗靶点。GITR 通过对 NLRP3 的翻译后调控,加剧了脓毒症中 LPC 诱导的巨噬细胞脓毒症。根据该模型,脓毒症早期 LPC 水平升高,诱导巨噬细胞表达 GITR。GITR 不仅会与 NLRP3 竞争与 E3 连接酶 MARCH7 的结合,还会招募 MARCH7 诱导去乙酰化酶 SIRT2 的降解,导致 NLRP3 的泛素化减少而乙酰化增加,从而加剧 LPC 诱导的 NLRP3 炎症小体激活、巨噬细胞脓毒症和全身炎症损伤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
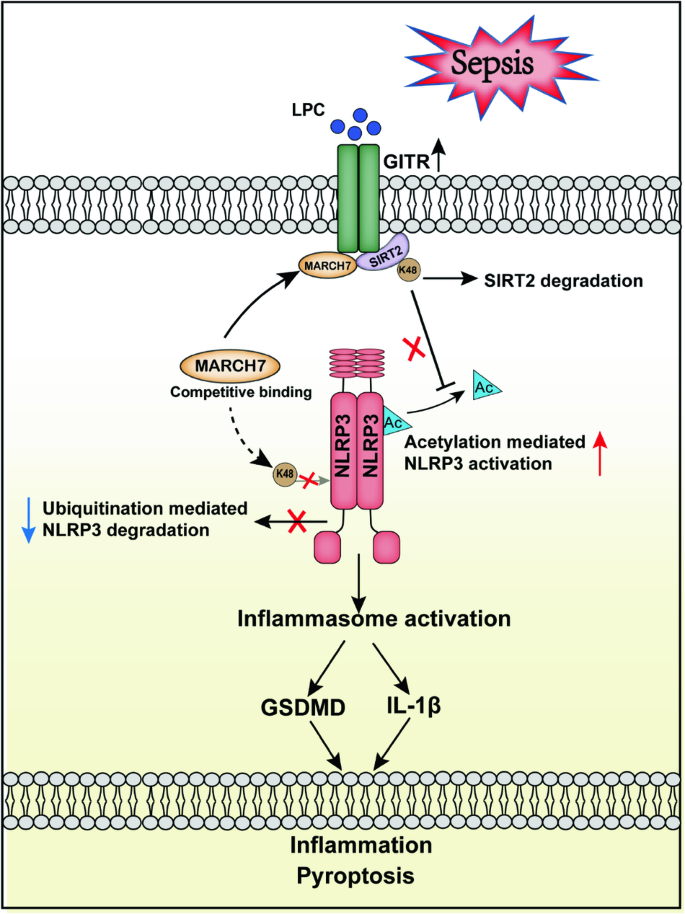
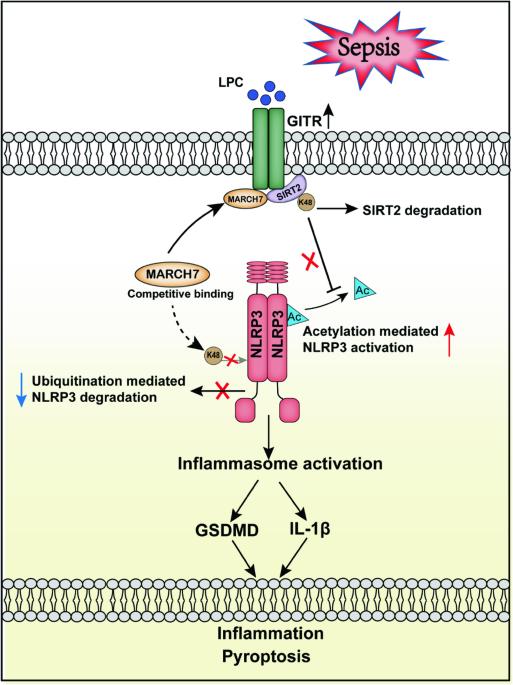
GITR exacerbates lysophosphatidylcholine-induced macrophage pyroptosis in sepsis via posttranslational regulation of NLRP3
The NLRP3 inflammasome functions as an inflammatory driver, but its relationship with lipid metabolic changes in early sepsis remains unclear. Here, we found that GITR expression in monocytes/macrophages was induced by lysophosphatidylcholine (LPC) and was positively correlated with the severity of sepsis. GITR is a costimulatory molecule that is mainly expressed on T cells, but its function in macrophages is largely unknown. Our in vitro data showed that GITR enhanced LPC uptake by macrophages and specifically enhanced NLRP3 inflammasome-mediated macrophage pyroptosis. Furthermore, in vivo studies using either cecal ligation and puncture (CLP) or LPS-induced sepsis models demonstrated that LPC exacerbated sepsis severity/lethality, while conditional knockout of GITR in myeloid cells or NLRP3/caspase-1/IL-1β deficiency attenuated sepsis severity/lethality. Mechanistically, GITR specifically enhanced inflammasome activation by regulating the posttranslational modification (PTM) of NLRP3. GITR competes with NLRP3 for binding to the E3 ligase MARCH7 and recruits MARCH7 to induce deacetylase SIRT2 degradation, leading to decreasing ubiquitination but increasing acetylation of NLRP3. Overall, these findings revealed a novel role of macrophage-derived GITR in regulating the PTM of NLRP3 and systemic inflammatory injury, suggesting that GITR may be a potential therapeutic target for sepsis and other inflammatory diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


