Eomesodermin通过分别靶向KLF2和T-bet,在时空上协调NK细胞发育的早期和晚期阶段。
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
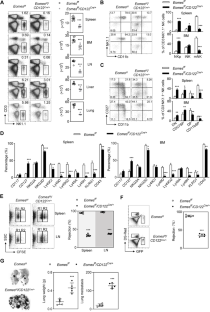
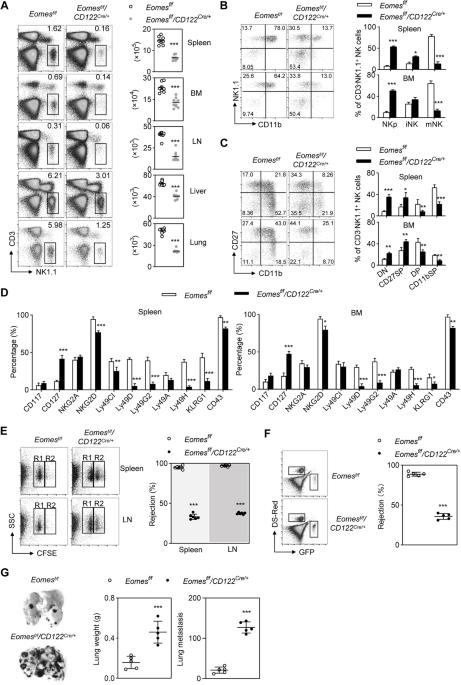
Eomesodermin(Eomes)是天然杀伤细胞(NK)发育过程中的一个关键因素,但它在这一过程中的时间和空间协调方面的确切作用仍不清楚。我们的研究发现,Eomes在NK细胞发育的早期和晚期发挥着不同的作用。具体来说,通过CD122-Cre转基因早期删除Eomes会导致另一个重要转录因子KLF2下调,从而在祖细胞阶段造成显著阻滞。ChIP-seq 发现,Eomes 与 Klf2 的保守非编码序列 (CNS) 直接结合。利用嵌合 IMmune 编辑(CHIME)技术,我们发现通过 CRISPRi 删除 Klf2 的 CNS 区域会导致 NK 细胞数量减少和发育停滞。此外,通过CRISPRa对这一特异性CNS区域进行组成性激活,可显著逆转Eomes缺乏导致的NK细胞发育严重缺陷。相反,Ncr1-Cre介导的Eomes末端缺失加速了NK细胞亚群从CD27+CD11b+表型向CD27-CD11b+表型的转变。编码 T-bet 的 Tbx21 的一个等位基因的基因缺失有效逆转了 Eomes 缺失的 NK 细胞的异常分化。总之,我们利用两种创新的遗传模型阐明了Eomes介导的NK细胞承诺和分化的复杂机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Eomesodermin spatiotemporally orchestrates the early and late stages of NK cell development by targeting KLF2 and T-bet, respectively
Eomesodermin (Eomes) is a critical factor in the development of natural killer (NK) cells, but its precise role in temporal and spatial coordination during this process remains unclear. Our study revealed that Eomes plays distinct roles during the early and late stages of NK cell development. Specifically, the early deletion of Eomes via the CD122-Cre transgene resulted in significant blockade at the progenitor stage due to the downregulation of KLF2, another important transcription factor. ChIP-seq revealed direct binding of Eomes to the conserved noncoding sequence (CNS) of Klf2. Utilizing the CHimeric IMmune Editing (CHIME) technique, we found that deletion of the CNS region of Klf2 via CRISPRi led to a reduction in the NK cell population and developmental arrest. Moreover, constitutive activation of this specific CNS region through CRISPRa significantly reversed the severe defects in NK cell development caused by Eomes deficiency. Conversely, Ncr1-Cre-mediated terminal deletion of Eomes expedited the transition of NK cell subsets from the CD27+CD11b+ phenotype to the CD27−CD11b+ phenotype. Late-stage deficiency of Eomes led to a significant increase in T-bet expression, which subsequently increased the expression of the transcription factor Zeb2. Genetic deletion of one allele of Tbx21, encoding T-bet, effectively reversed the aberrant differentiation of Eomes-deficient NK cells. In summary, we utilized two innovative genetic models to elucidate the intricate mechanisms underlying Eomes-mediated NK cell commitment and differentiation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


