Baoyue Pan, Shumei Yan, Linjing Yuan, Huiling Xiang, Mingxiu Ju, Shijie Xu, Weihua Jia, Jundong Li, Qi Zhao, Min Zheng
下载PDF
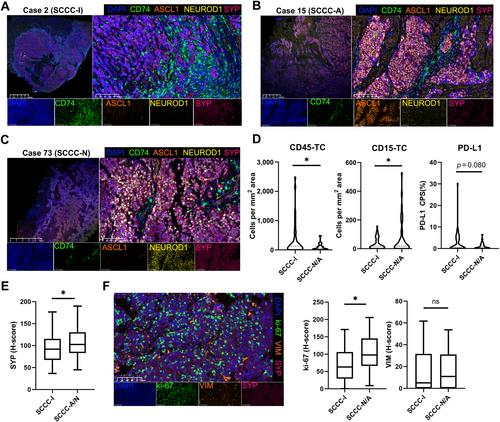
{"title":"多组学测序和免疫微环境特征确定了宫颈小细胞神经内分泌癌的三种亚型。","authors":"Baoyue Pan, Shumei Yan, Linjing Yuan, Huiling Xiang, Mingxiu Ju, Shijie Xu, Weihua Jia, Jundong Li, Qi Zhao, Min Zheng","doi":"10.1002/path.6290","DOIUrl":null,"url":null,"abstract":"<p>Small cell cervical carcinoma (SCCC) is the most common neuroendocrine tumor in the female genital tract, with an unfavorable prognosis and lacking an evidence-based therapeutic approach. Until now, the distinct subtypes and immune characteristics of SCCC combined with genome and transcriptome have not been described. We performed genomic (<i>n</i> = 18), HPV integration (<i>n</i> = 18), and transcriptomic sequencing (<i>n</i> = 19) of SCCC samples. We assessed differences in immune characteristics between SCCC and conventional cervical cancer, and other small cell neuroendocrine carcinomas, through bioinformatics analysis and immunohistochemical assays. We stratified SCCC patients through non-negative matrix factorization and described the characteristics of these distinct types. We further validated it using multiplex immunofluorescence (<i>n</i> = 77) and investigated its clinical prognostic effect. We confirmed a high frequency of <i>PIK3CA</i> and <i>TP53</i> alterations and HPV18 integrations in SCCC. SCCC and other small cell carcinoma had similar expression signatures and immune cell infiltration patterns. Comparing patients with SCCC to those with conventional cervical cancer, the former presented immune excluded or ‘desert’ infiltration. The number of CD8+ cells in the invasion margin of SCCC patients predicted favorable clinical outcomes. We identified three transcriptome subtypes: an inflamed phenotype with high-level expression of genes related to the MHC-II complex (<i>CD74</i>) and IFN-α/β (SCCC-I), and two neuroendocrine subtypes with high-level expression of <i>ASCL1</i> or <i>NEUROD1</i>, respectively. Combined with multiple technologies, we found that the neuroendocrine groups had more <i>TP53</i> mutations and SCCC-I had more <i>PIK3CA</i> mutations. Multiplex immunofluorescence validated these subtypes and SCCC-I was an independent prognostic factor of overall survival. These results provide insights into SCCC tumor heterogeneity and potential therapies. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 3","pages":"372-385"},"PeriodicalIF":5.2000,"publicationDate":"2024-05-09","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6290","citationCount":"0","resultStr":"{\"title\":\"Multiomics sequencing and immune microenvironment characteristics define three subtypes of small cell neuroendocrine carcinoma of the cervix\",\"authors\":\"Baoyue Pan, Shumei Yan, Linjing Yuan, Huiling Xiang, Mingxiu Ju, Shijie Xu, Weihua Jia, Jundong Li, Qi Zhao, Min Zheng\",\"doi\":\"10.1002/path.6290\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Small cell cervical carcinoma (SCCC) is the most common neuroendocrine tumor in the female genital tract, with an unfavorable prognosis and lacking an evidence-based therapeutic approach. Until now, the distinct subtypes and immune characteristics of SCCC combined with genome and transcriptome have not been described. We performed genomic (<i>n</i> = 18), HPV integration (<i>n</i> = 18), and transcriptomic sequencing (<i>n</i> = 19) of SCCC samples. We assessed differences in immune characteristics between SCCC and conventional cervical cancer, and other small cell neuroendocrine carcinomas, through bioinformatics analysis and immunohistochemical assays. We stratified SCCC patients through non-negative matrix factorization and described the characteristics of these distinct types. We further validated it using multiplex immunofluorescence (<i>n</i> = 77) and investigated its clinical prognostic effect. We confirmed a high frequency of <i>PIK3CA</i> and <i>TP53</i> alterations and HPV18 integrations in SCCC. SCCC and other small cell carcinoma had similar expression signatures and immune cell infiltration patterns. Comparing patients with SCCC to those with conventional cervical cancer, the former presented immune excluded or ‘desert’ infiltration. The number of CD8+ cells in the invasion margin of SCCC patients predicted favorable clinical outcomes. We identified three transcriptome subtypes: an inflamed phenotype with high-level expression of genes related to the MHC-II complex (<i>CD74</i>) and IFN-α/β (SCCC-I), and two neuroendocrine subtypes with high-level expression of <i>ASCL1</i> or <i>NEUROD1</i>, respectively. Combined with multiple technologies, we found that the neuroendocrine groups had more <i>TP53</i> mutations and SCCC-I had more <i>PIK3CA</i> mutations. Multiplex immunofluorescence validated these subtypes and SCCC-I was an independent prognostic factor of overall survival. These results provide insights into SCCC tumor heterogeneity and potential therapies. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"263 3\",\"pages\":\"372-385\"},\"PeriodicalIF\":5.2000,\"publicationDate\":\"2024-05-09\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6290\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.6290\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.6290","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用



 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


