用于恶性疟原虫 DNA 转染的人红细胞质粒装载优化。
IF 3.7
2区 医学
Q1 PARASITOLOGY
引用次数: 0
摘要
恶性疟原虫基因的体外改造是疟疾基础研究和转化研究的基石。通过 DNA 转染,这些修饰可能会改变蛋白质序列或丰度。此类实验对于确定关键寄生虫表型的分子机制以及验证药物和疫苗靶点至关重要。尽管转染很重要,但成功转染仍然很困难,是恶性疟原虫研究中一个资源密集型的限制性步骤。在此,我们报告了质粒在红细胞中的低效负载限制了常用电穿孔方法的转染效果。由于这些方法还需要昂贵的仪器和耗材,而这些仪器和耗材并不能广泛使用,因此我们探索了一种更简单的方法,即通过低渗裂解红细胞并重新封口来装载质粒。我们利用寄生虫表达敏感的 NanoLuc 报告器来快速评估和优化每个步骤。低渗缓冲液的成分、再封闭缓冲液的容量和成分以及随后的培养都会影响质粒的保留和成功转染。虽然 ATP 对红细胞再封闭至关重要,但添加 Ca++ 或谷胱甘肽并不能提高转染效率,Ca++ 浓度的增加对转染结果不利。与标准电穿孔方法或之前报道的低渗装载方案相比,优化方法能在转染 48 小时后产生更多的质粒装载和更高的 NanoLuc 报告基因表达。在利用外显子表达或 CRISPR-Cas9 介导的整合进行转染时,它还能明显加快寄生虫的生长速度。这种新方法可提高恶性疟原虫的转染效率,减少资源需求,并可加速疟疾药物和疫苗靶标的分子研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
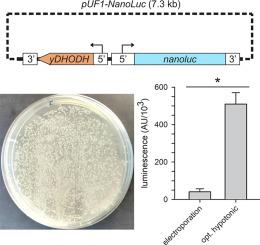
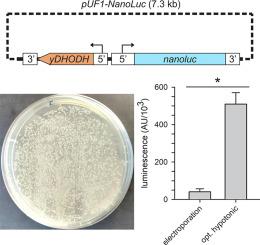
Optimized plasmid loading of human erythrocytes for Plasmodium falciparum DNA transfections
In vitro modification of Plasmodium falciparum genes is the cornerstone of basic and translational malaria research. Achieved through DNA transfection, these modifications may entail altering protein sequence or abundance. Such experiments are critical for defining the molecular mechanisms of key parasite phenotypes and for validation of drug and vaccine targets. Despite its importance, successful transfection remains difficult and is a resource-intensive, rate-limiting step in P. falciparum research. Here, we report that inefficient loading of plasmid into erythrocytes limits transfection efficacy with commonly used electroporation methods. As these methods also require expensive instrumentation and consumables that are not broadly available, we explored a simpler method based on plasmid loading through hypotonic lysis and resealing of erythrocytes. We used parasite expression of a sensitive NanoLuc reporter for rapid evaluation and optimization of each step. Hypotonic buffer composition, resealing buffer volume and composition, and subsequent incubation affected plasmid retention and successful transfection. While ATP was critical for erythrocyte resealing, addition of Ca++ or glutathione did not improve transfection efficiency, with increasing Ca++ concentrations proving detrimental to outcomes. Compared with either the standard electroporation method or a previously reported hypotonic loading protocol, the optimized method yields greater plasmid loading and higher expression of the NanoLuc reporter 48 h after transfection. It also produced significantly faster outgrowth of parasites in transfections utilizing either episomal expression or CRISPR-Cas9 mediated integration. This new method produces higher P. falciparum transfection efficiency, reduces resource requirements and should accelerate molecular studies of malaria drug and vaccine targets.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
2.50%
发文量
76
审稿时长
23 days
期刊介绍:
International Journal for Parasitology offers authors the option to sponsor nonsubscriber access to their articles on Elsevier electronic publishing platforms. For more information please view our Sponsored Articles page. The International Journal for Parasitology publishes the results of original research in all aspects of basic and applied parasitology, including all the fields covered by its Specialist Editors, and ranging from parasites and host-parasite relationships of intrinsic biological interest to those of social and economic importance in human and veterinary medicine and agriculture.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


