肌动蛋白结合蛋白 CAP1 可抑制小鼠大脑皮层中 MRTF-SRF 依赖性基因的表达。
IF 6.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
血清反应因子(SRF)是大脑发育和功能所必需的转录因子。在这里,我们探讨了SRF辅助因子--肌动蛋白单体感应肌动蛋白相关转录因子MRTF如何在小鼠皮质神经元中受到调控。我们发现,体外和体内依赖于 MRTF 的 SRF 活性受到环化酶相关蛋白 CAP1 的抑制。肌动蛋白结合蛋白CAP1的失活减少了细胞质中肌动蛋白单体的数量,从而促进了核MRTF的转位和MRTF-SRF的激活。这一功能独立于cofilin1和肌动蛋白解聚因子,而且皮质神经元中CAP1功能的缺失不会被内源性CAP2所补偿。对野生型小鼠和Cap1基因敲除小鼠大脑皮层裂解物的转录组和蛋白质组分析证实了CAP1在体内抑制MRTF-SRF依赖性信号传导中的作用。生物信息学分析确定了可能的 MRTF-SRF 靶基因,这与转录组和蛋白质组的结果一致。我们之前的研究表明 CAP1 与轴突生长锥的功能以及兴奋性突触的形态和可塑性有关,结合这些研究结果,我们的发现确立了 CAP1 是大脑中与神经元网络的形成有关的关键肌动蛋白调节因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
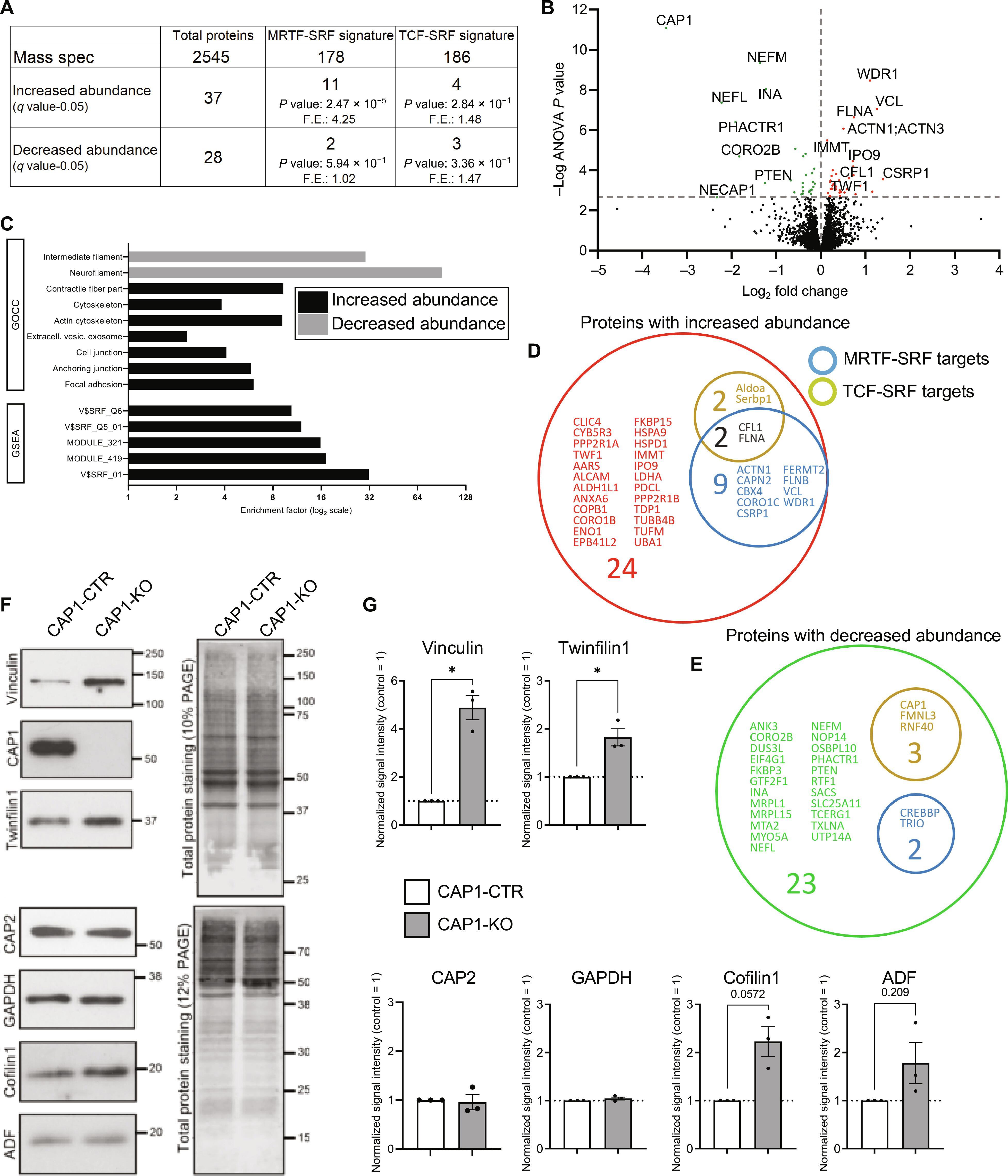
The actin-binding protein CAP1 represses MRTF-SRF–dependent gene expression in mouse cerebral cortex
Serum response factor (SRF) is an essential transcription factor for brain development and function. Here, we explored how an SRF cofactor, the actin monomer-sensing myocardin-related transcription factor MRTF, is regulated in mouse cortical neurons. We found that MRTF-dependent SRF activity in vitro and in vivo was repressed by cyclase-associated protein CAP1. Inactivation of the actin-binding protein CAP1 reduced the amount of actin monomers in the cytoplasm, which promoted nuclear MRTF translocation and MRTF-SRF activation. This function was independent of cofilin1 and actin-depolymerizing factor, and CAP1 loss of function in cortical neurons was not compensated by endogenous CAP2. Transcriptomic and proteomic analyses of cerebral cortex lysates from wild-type and Cap1 knockout mice supported the role of CAP1 in repressing MRTF-SRF–dependent signaling in vivo. Bioinformatic analysis identified likely MRTF-SRF target genes, which aligned with the transcriptomic and proteomic results. Together with our previous studies that implicated CAP1 in axonal growth cone function as well as the morphology and plasticity of excitatory synapses, our findings establish CAP1 as a crucial actin regulator in the brain relevant for formation of neuronal networks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


