低剂量华法林与新型机械主动脉瓣:随访 5 年的中期登记结果。
IF 4.9
1区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
Journal of Thoracic and Cardiovascular Surgery
Pub Date : 2024-12-01
DOI:10.1016/j.jtcvs.2024.04.017
引用次数: 0
摘要
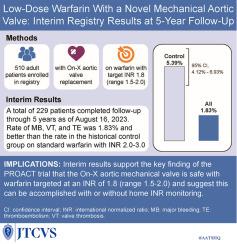
目的评估所有患者在接受 On-X 机械主动脉瓣植入术后,以国际正常化比率 1.8(范围 1.5-2.0)为目标的华法林是否安全:这项前瞻性、观察性临床登记评估了接受小剂量华法林(目标国际正常化比率 1.8,范围 1.5-2.0)加每日阿司匹林(75-100 毫克)治疗的成年患者在 On-X 主动脉瓣植入术后 5 年内的不良事件发生率。主要终点是总体和 4 个亚组的大出血、瓣膜血栓和血栓栓塞综合发生率。对比组为前瞻性随机 On-X 抗凝试验对照组,患者每天服用标准剂量华法林(国际正常化比率 2.0-3.0)加阿司匹林 81 毫克:2015年11月至2022年1月期间,美国、英国和加拿大的23个中心共招募了510名患者。本次中期分析包括计划在2023年8月16日前完成5年随访的229名患者。大出血、瓣膜血栓形成和血栓栓塞等主要复合终点的线性化发生率(每患者年百分比)为 1.83%,而对比组为 5.39%(95% 置信区间为 4.12%-6.93%)。诊所监测组和家庭监测组以及血栓栓塞高危患者的结果一致。与对照组相比,大出血和总出血量分别减少了87%和71%,但血栓栓塞事件并未增加:中期研究结果表明,无论是否进行家庭监测,On-X 主动脉瓣机械瓣膜在植入后 5 年内,国际正常化比率目标值为 1.8,并服用小剂量阿司匹林,仍具有持续的安全性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Low-dose warfarin with a novel mechanical aortic valve: Interim registry results at 5-year follow-up
Objectives
To evaluate whether warfarin targeted at an international normalized ratio of 1.8 (range, 1.5-2.0) after On-X mechanical aortic valve implant is safe for all patients.
Methods
This prospective, observational clinical registry assessed adverse event rates in adult patients receiving low-dose warfarin (target international normalized ratio, 1.8; range, 1.5-2.0) plus daily aspirin (75-100 mg) during a 5-year period after On-X aortic valve implant. The primary end point is the combined rate of major bleeding, valve thrombosis, and thromboembolism overall and in 4 subgroups. The comparator is the Prospective Randomized On-X Anticoagulation Trial control group patients on standard-dose warfarin (international normalized ratio, 2.0-3.0) plus aspirin 81 milligrams daily.
Results
A total of 510 patients were recruited at 23 centers in the United States, United Kingdom, and Canada between November 2015 and January 2022. This interim analysis includes 229 patients scheduled to complete 5-year follow-up by August 16, 2023. The linearized occurrence rate (in percent per patient-year) of the primary composite end point of major bleeding, valve thrombosis, and thromboembolism is 1.83% compared with 5.39% (95% confidence interval, 4.12%-6.93%) in the comparator group. Results are consistent in clinic-monitored and home-monitored patients and in those at high risk for thromboembolism. Major bleeding and total bleeding were reduced by 87% and 71%, respectively, versus the comparator group, without an increase in thromboembolic events.
Conclusions
Interim results support the continued safety of the On-X aortic mechanical valve with a target international normalized ratio of 1.8 plus low-dose aspirin through 5 years after implant, with or without home monitoring.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.20
自引率
10.00%
发文量
1079
审稿时长
68 days
期刊介绍:
The Journal of Thoracic and Cardiovascular Surgery presents original, peer-reviewed articles on diseases of the heart, great vessels, lungs and thorax with emphasis on surgical interventions. An official publication of The American Association for Thoracic Surgery and The Western Thoracic Surgical Association, the Journal focuses on techniques and developments in acquired cardiac surgery, congenital cardiac repair, thoracic procedures, heart and lung transplantation, mechanical circulatory support and other procedures.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


