应变促进的炔烃-硝酮环加成(SPANC)与无保护碳水化合物支架化硝基的动力学研究
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
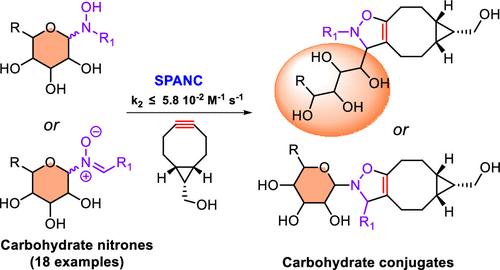
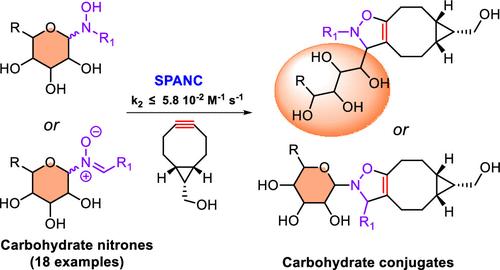
我们研究了在菌株促进的炔-硝酮环加成反应中使用未受保护的碳水化合物衍生的亚硝基作为合作伙伴,以此作为生物共轭的一种新工具。观察到的二阶反应显示出 3.4 × 10-4-5.8 × 10-2 M-1 s-1 的速率常数,这是与其他简单脂肪族或芳香族亚硝基化合物反应动力学的共同数量级。这种方法适用于水性介质,具体方法是在水中进行单锅反应,将腈酮的连续生成和与环辛炔的环化反应结合起来。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kinetics of Strain-Promoted Alkyne-Nitrone Cycloadditions (SPANC) with Unprotected Carbohydrate Scaffolded Nitrones
The use of unprotected carbohydrate-derived nitrones as partners in strain-promoted alkyne-nitrone cycloadditions was investigated as a new tool for bioconjugation. The observed second-order reactions displayed rate constants of 3.4 × 10–4–5.8 × 10–2 M–1 s–1, which is the common order of magnitude of reaction kinetics with other simple aliphatic or aromatic nitrones. Applicability of this method to aqueous media was demonstrated by performing a one-pot protocol, which combines sequential formation of the nitrone and cycloaddition with cyclooctyne in water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


