无机 α 亲核物在水介质和微胶囊介质中酰基转移中的反应性:IV.有组织微异质体系中酰基衍生物的过氧化水解1
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
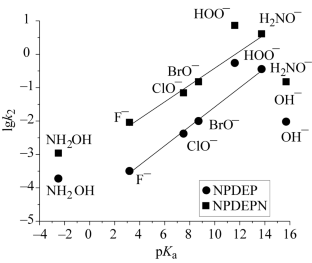
摘要在基于双阳离子[双子表面活性剂 AlkIm+-(CH2)3-Im+Alk∙2Br-, 其中 Alk = C12H25 或 C14H29 (GS)] 和单阳离子[AlkIm+CH3∙Br-, 其中 Alk = C12H25 或 C14H29 (GS)] 的有组织微异构体系中,磷酸、膦酸和甲苯磺酸的 4-硝基苯基酯的过水解和碱催化水解的胶束效应、其中 Alk=C12H25 或 C14H29 (GS)] 和单配位(AlkIm+CH3∙Br-,其中 Alk=C12H25 或 C14H29)表面活性剂的有组织微均质系统进行了分析。结果发现,试剂浓度是导致胶束催化作用的主要因素。过氧化氢 α 效应(定义为过水解和碱催化水解的二阶速率常数之比)得以保留,并且根据表面活性剂和底物的性质,可达到约 100。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Reactivity of Inorganic α-Nucleophiles in Acyl Transfer in Aqueous and Micellar Media: IV. Peroxyhydrolysis of Acyl Derivatives in Organized Microheterogeneous Systems1
The micellar effects in the perhydrolysis and base-catalyzed hydrolysis of 4-nitrophenyl esters of phosphoric, phosphonic, and toluenesulfonic acids in organized microheterogeneous systems based on dicationic [Gemini surfactant AlkIm+–(CH2)3–Im+Alk∙2Br–, where Alk = C12H25 or C14H29 (GS)] and monocationic (AlkIm+CH3∙Br–, where Alk=C12H25 or C14H29) surfactants have been analyzed. The reagent concentrations have been found to be the main factor responsible for micellar catalysis. The hydroperoxide α-effect defined as the ratio of the second-order rate constants of perhydrolysis and base-catalyzed hydrolysis is preserved and, depending on the nature of the surfactant and the substrate, may reach ~ 100.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


