深共晶溶剂诱导可控合成用于电化学水分离的双功能 Ni-Fe-P 催化剂
IF 7.6
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
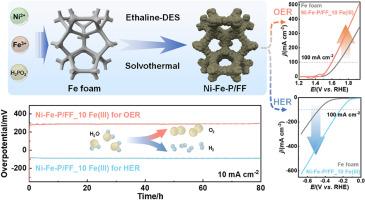
开发具有优异活性、高稳定性和低成本的电催化剂对于通过电化学水分离大规模制氢至关重要。在此,我们在乙二醇和氯化胆碱的深共晶溶剂(DES)(命名为乙酞)中,通过简单的一步溶剂热法,开发出了一种在铁泡沫上原位生长的双功能镍-芴-P 催化剂(镍-芴-P/FF)。乙酞的独特溶剂环境以及引入的铁(III)离子的调节作用在制备过程中发挥了重要作用。所开发的 Ni-Fe-P/FF 可作为高效的双功能电催化剂在 1.0 M KOH 中进行水分离,氧气和氢气进化反应分别需要 82 mV (229 mV) 和 263 mV (370 mV) 的过电位才能达到 10 mA cm-2 (100 mA cm-2)。此外,自支撑催化剂组装的电解槽也表现出良好的催化性能,只需 1.83 V 的低电压即可驱动 100 mA cm-2 的反应,并且在 100 小时内具有良好的稳定性。这项工作为制造催化水分离的高性能双功能 Ni-Fe-P 电催化剂提供了一种简便的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Deep eutectic solvent-induced controllable synthesis of bifunctional Ni–Fe–P catalysts for electrochemical water splitting
Developing electrocatalysts with excellent activity, high stability, and low cost is vital for large-scale hydrogen production through electrochemical water splitting. Herein, a bifunctional Ni–Fe–P catalyst in situ grown on Fe foam (Ni–Fe–P/FF) is developed by a simple one-step solvothermal process in the deep eutectic solvent (DES) of ethylene glycol and choline chloride (named Ethaline). The unique solvent environment of Ethaline assisted with the regulating effect of the introduced Fe(III) ions shows an essential role in governing the preparation process. The developed Ni–Fe–P/FF acts as the efficient bifunctional electrocatalyst for water splitting in 1.0 M KOH, requiring overpotentials of 82 mV (229 mV) and 263 mV (370 mV) to deliver 10 mA cm−2 (100 mA cm−2) for oxygen and hydrogen evolution reactions, respectively. Furthermore, the self-supported catalyst-assembled electrolyzer also exhibits good catalytic performance with a low voltage of 1.83 V to drive 100 mA cm−2 and good stability over 100 h. This work offers a facile approach to fabricating high-performance bifunctional Ni–Fe–P electrocatalysts to catalyze water splitting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemical Engineering
Process Chemistry and Technology, Catalysis, Filtration and Separation
CiteScore
11.60
自引率
0.00%
发文量
58
审稿时长
51 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


