评估全球药品和医疗器械行业结构化效益-风险评估现状的调查。
IF 2
4区 医学
Q4 MEDICAL INFORMATICS
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
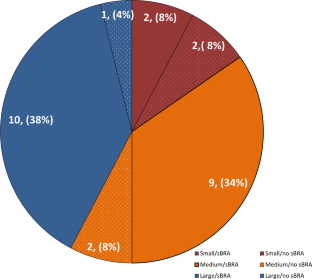
A Survey to Assess the Current Status of Structured Benefit-Risk Assessment in the Global Drug and Medical Device Industry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Therapeutic innovation & regulatory science
MEDICAL INFORMATICS-PHARMACOLOGY & PHARMACY
CiteScore
3.40
自引率
13.30%
发文量
127
期刊介绍:
Therapeutic Innovation & Regulatory Science (TIRS) is the official scientific journal of DIA that strives to advance medical product discovery, development, regulation, and use through the publication of peer-reviewed original and review articles, commentaries, and letters to the editor across the spectrum of converting biomedical science into practical solutions to advance human health.
The focus areas of the journal are as follows:
Biostatistics
Clinical Trials
Product Development and Innovation
Global Perspectives
Policy
Regulatory Science
Product Safety
Special Populations
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


