卤素-键促进 3-溴-3-烷基-2,2-二氟丙酸乙酯与香豆素/喹啉酮的直接交叉偶联反应
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们开发了一种实用的方法,利用卤素键作为关键的非共价相互作用,直接将 3-溴-3-烷基-2,2-二氟丙酸乙酯与香豆素/喹啉酮进行交叉偶联。利用这一方案,我们在温和的反应条件下成功合成了结构多样的二氟烷基化香豆素和喹啉酮衍生物库,并获得了中等至良好的产率。值得注意的是,化合物 3l 对 Huh-7 和 A549 癌细胞株都有很强的抗肿瘤功效,其 IC50 值分别为 4.6 μM 和 6.8 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
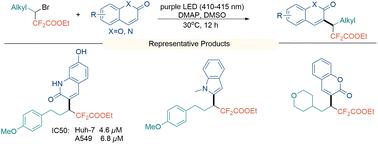
Halogen-bond-promoted direct cross-coupling of ethyl 3-bromo-3-alkyl-2,2-difluoropropanoates with coumarins/quinolinones†
Herein, we developed a practical method for the direct cross-coupling of ethyl 3-bromo-3-alkyl-2,2-difluoropropanoates with coumarins/quinolinones, leveraging a halogen bond as the pivotal non-covalent interaction. Employing this protocol, a library of structurally diverse difluoroalkylated coumarin and quinolinone derivatives has been successfully synthesized under mild reaction conditions, affording moderate to good yields. Notably, compound 3l demonstrated potent antitumor efficacy against both Huh-7 and A549 cancer cell lines, with IC50 values of 4.6 μM and 6.8 μM, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


