Lkb1 协调γδ T 细胞的代谢和功能,控制 IL-17 介导的自身免疫性肝炎
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
γδT细胞在免疫监视中起着至关重要的作用,是先天性免疫和适应性免疫之间的桥梁。然而,人们对γδ T细胞发育和功能的代谢要求和调控仍然知之甚少。在这项研究中,我们研究了肝激酶 B1(Lkb1)在γδ T 细胞生物学中的作用,肝激酶 B1 是一种丝氨酸/苏氨酸激酶,它将细胞代谢与细胞生长和增殖联系在一起。我们的研究结果表明,Lkb1 不仅参与调节γδ T 细胞系的承诺,而且在γδ T 细胞效应功能中发挥着关键作用。具体来说,T 细胞特异性缺失 Lkb1 会导致胸腺细胞发育受损,胸腺和外周淋巴组织中的γδ T 细胞亚群发生明显改变。值得注意的是,Lkb1 的缺失抑制了 Vγ1 和 Vγ4 γδ T 细胞的承诺,促进了产生 IL-17 的 Vγ6 γδ T 细胞的成熟,并导致致命的自身免疫性肝炎(AIH)的发生。值得注意的是,清除γδT细胞或阻断IL-17可显著减轻AIH。从机理上讲,Lkb1 缺乏会破坏代谢平衡和 AMPK 活性,同时增加 mTORC1 的活化,从而导致γδ T 细胞过度活化和细胞凋亡增强。有趣的是,激活 AMPK 或抑制 mTORC1 信号传导可有效抑制 IL-17 水平并减轻 Lkb1 基因缺陷小鼠的 AIH。我们的研究结果突显了Lkb1在维持γδT细胞平衡和预防IL-17介导的自身免疫性疾病中的关键作用,为研究胸腺γδT细胞亚群确定和功能分化的代谢程序提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lkb1 orchestrates γδ T-cell metabolic and functional fitness to control IL-17-mediated autoimmune hepatitis
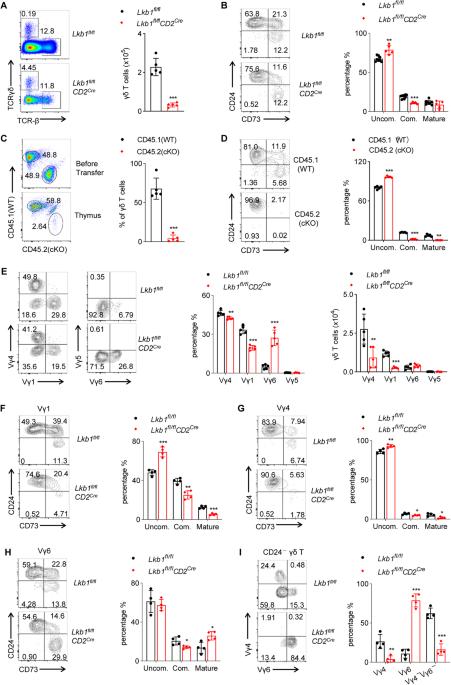
γδ T cells play a crucial role in immune surveillance and serve as a bridge between innate and adaptive immunity. However, the metabolic requirements and regulation of γδ T-cell development and function remain poorly understood. In this study, we investigated the role of liver kinase B1 (Lkb1), a serine/threonine kinase that links cellular metabolism with cell growth and proliferation, in γδ T-cell biology. Our findings demonstrate that Lkb1 is not only involved in regulating γδ T lineage commitment but also plays a critical role in γδ T-cell effector function. Specifically, T-cell-specific deletion of Lkb1 resulted in impaired thymocyte development and distinct alterations in γδ T-cell subsets in both the thymus and peripheral lymphoid tissues. Notably, loss of Lkb1 inhibited the commitment of Vγ1 and Vγ4 γδ T cells, promoted the maturation of IL-17-producing Vγ6 γδ T cells, and led to the occurrence of fatal autoimmune hepatitis (AIH). Notably, clearance of γδ T cells or blockade of IL-17 significantly attenuated AIH. Mechanistically, Lkb1 deficiency disrupted metabolic homeostasis and AMPK activity, accompanied by increased mTORC1 activation, thereby causing overactivation of γδ T cells and enhanced apoptosis. Interestingly, activation of AMPK or suppression of mTORC1 signaling effectively inhibited IL-17 levels and attenuated AIH in Lkb1-deficient mice. Our findings highlight the pivotal role of Lkb1 in maintaining the homeostasis of γδ T cells and preventing IL-17-mediated autoimmune diseases, providing new insights into the metabolic programs governing the subset determination and functional differentiation of thymic γδ T cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


