以美托洛尔为底物药物,利用药代动力学和药效学模型研究新型 CYP2D6 变体的基因型和表型
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
摘要
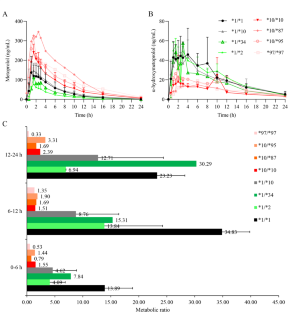
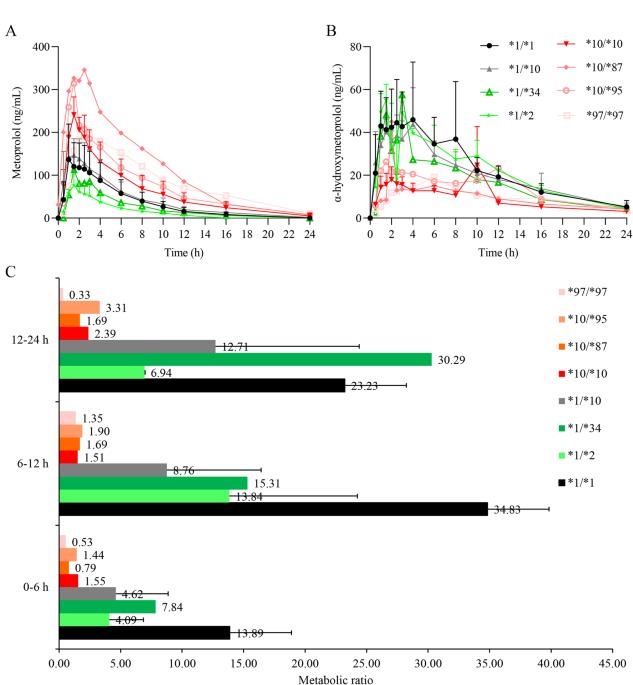
为了研究携带 CYP2D6 基因型且代谢表型未知的志愿者的药代动力学和药效学特征,我们根据测序结果招募了 22 名志愿者。在口服美托洛尔后的特定时间点采集外周血和尿液样本。采用经过验证的高效液相色谱法(HPLC)测定美托洛尔和α-羟基美托洛尔的浓度。同时还对血压和心电图进行了监测。结果显示,CYP2D6*1/*34携带者体内美托洛尔的主要药代动力学参数与CYP2D6*1/*1携带者相似。然而,与野生型相比,CYP2D6*10/*87、CYP2D6*10/*95 和 CYP2D6*97/*97 基因型携带者体内美托洛尔的曲线下面积(AUC)和半衰期(t1/2)增加了 2-3 倍。这些基因型中美托洛尔的尿代谢比率与血浆样本中观察到的趋势一致。因此,CYP2D6*1/*34 可被视为正常代谢者,而 CYP2D6*10/*87、CYP2D6*10/*95 和 CYP2D6*97/*97 则是中间代谢者。尽管研究发现美托洛尔的血药浓度与 CYP2D6 基因型有关,但其降压效果在血压降低 25 mmHg 时达到最大。此外,P-Q 间期延长和心率降低与美托洛尔的血药浓度并不呈正相关。基于药代动力学-药效学模型,该研究阐明了美托洛尔在新型 CYP2D6 基因型受试者中的特性,为该底物药物的转化医学提供了重要的基础数据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Study on genotype and phenotype of novel CYP2D6 variants using pharmacokinetic and pharmacodynamic models with metoprolol as a substrate drug
To investigate the pharmacokinetic and pharmacodynamic profiles of volunteers carrying CYP2D6 genotypes with unknow metabolic phenotypes, a total of 22 volunteers were recruited based on the sequencing results. Peripheral blood and urine samples were collected at specific time points after oral administration of metoprolol. A validated high-performance liquid chromatography (HPLC) method was used to determine the concentrations of metoprolol and α-hydroxymetoprolol. Blood pressure and electrocardiogram were also monitored. The results showed that the main pharmacokinetic parameters of metoprolol in CYP2D6*1/*34 carriers are similar to those in CYP2D6*1/*1 carriers. However, in individuals carrying the CYP2D6*10/*87, CYP2D6*10/*95, and CYP2D6*97/*97 genotypes, the area under the curve (AUC) and half-life (t1/2) of metoprolol increased by 2-3 times compared to wild type. The urinary metabolic ratio of metoprolol in these genotypes is consistent with the trends observed in plasma samples. Therefore, CYP2D6*1/*34 can be considered as normal metabolizers, while CYP2D6*10/*87, CYP2D6*10/*95, and CYP2D6*97/*97 are intermediate metabolizers. Although the blood concentration of metoprolol has been found to correlate with CYP2D6 genotype, its blood pressure-lowering effect reaches maximum effectiveness at a reduction of 25 mmHg. Furthermore, P-Q interval prolongation and heart rate reduction are not positively correlated with metoprolol blood exposure. Based on the pharmacokinetic-pharmacodynamic model, this study clarified the properties of metoprolol in subjects with novel CYP2D6 genotypes and provided important fundamental data for the translational medicine of this substrate drug.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


