以 L-脯氨酸为基础的 DES 在 Knoevenagel 合成芳基阮丹宁、噻唑烷-2,4-二酮和巴比妥酸衍生物的过程中的应用
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
深共晶溶剂(DES)是一种避免使用有毒有机溶剂的环保型溶剂,近年来已得到广泛研究。然而,在加工和分离产物的过程中,仍经常会用到挥发性有机化合物(VOC)。在此,报告了一种用于克诺文纳格尔反应的零挥发性有机化合物策略。(在基于 L-脯氨酸的 DES 中,(杂)芳香醛与罗丹宁、噻唑烷-2,4-二酮 TZD 或巴比妥酸在温和条件下成功缩合,水解后无需任何纯化即可获得纯化合物。对于活性较低的 TZD,通过微波活化可将反应时间从 24 小时缩短到 1 小时。本文章由计算机程序翻译,如有差异,请以英文原文为准。
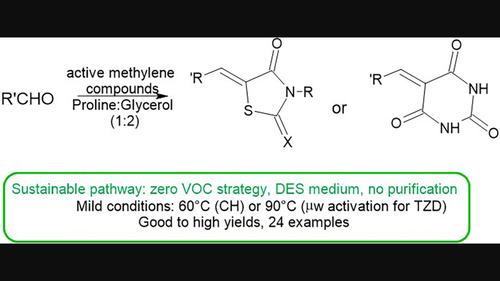
L-proline-based DES in Knoevenagel synthesis of arylidene rhodanines, thiazolidine-2,4-diones, and barbituric derivatives
Deep eutectic solvents (DES) are environmentally friendly solvents that prevent the use of toxic organic solvents and have been extensively studied in recent years. However, volatile organic compounds (VOC) are often still used during workup and isolation of products. Here, a zero-VOC strategy for Knoevenagel reaction is reported. (Hetero)aromatic aldehydes are successfully condensed with rhodanine, thiazolidine-2,4-dione TZD, or barbituric acid under mild conditions in an L-proline-based DES and pure compounds are obtained after hydrolysis without any purification. For the less reactive TZD, activation by microwave allows diminution of reaction time from 24 h to just 1 h.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


