预防性使用棘白菌素对接受含硫柳胺调理方案进行干细胞移植的患者出现神经系统并发症的影响:单中心回顾性研究
IF 1.2
4区 医学
Q3 PEDIATRICS
引用次数: 0
摘要
背景虽然神经毒性是与丁苯磺胺相关的主要不良事件,但有关丁苯磺胺治疗方案中药物相互作用与神经症状之间关系的信息却很少。本研究评估了干细胞移植患者在接受含丁苯磺胺的调理方案时,预防性使用棘白类药物与神经系统并发症之间的关联。方法我们回顾性地纳入了2007年至2022年期间在本院接受静脉注射丁苯磺胺作为调理方案的连续患者。预防性使用棘白菌素是指使用棘白菌素类抗真菌药物预防干细胞移植受者感染真菌疾病。主要研究结果是开始使用硫酸氢钠后7天内神经系统并发症的发生率,并在棘白菌素组(接受预防性棘白菌素治疗的患者)和非棘白菌素组(接受棘白菌素以外的预防性抗真菌药物治疗的患者和未接受抗真菌预防治疗的患者)之间进行比较。结果在纳入本研究的 59 名患者中,棘白菌素组(26 人)和非棘白菌素组(33 人)的神经系统并发症发生率分别为 30.8% 和 63.6%。在对接受预防性棘白菌素类药物的倾向评分进行调整后,我们发现预防性使用棘白菌素类药物与神经系统并发症的发生呈负相关(调整后的几率比为 0.294,95% 置信区间为 0.090 至 0.959)。我们观察到,使用棘白菌素类药物组的神经系统并发症发生率低于未使用棘白菌素类药物组。本文章由计算机程序翻译,如有差异,请以英文原文为准。
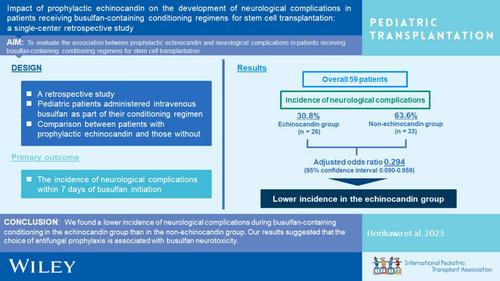
Impact of prophylactic echinocandin on the development of neurological complications in patients receiving busulfan‐containing conditioning regimens for stem cell transplantation: A single‐center retrospective study
BackgroundAlthough neurotoxicity is a major adverse event associated with busulfan, little information is available regarding the association between drug interactions and neurological symptoms during busulfan‐based regimens. This study evaluated the association between prophylactic echinocandins and neurological complications in patients receiving busulfan‐containing conditioning regimens for stem cell transplantation.MethodsWe retrospectively included consecutive patients who administered intravenous busulfan as a conditioning regimen at our facility between 2007 and 2022. Prophylactic echinocandin use was defined as the use of an echinocandin antifungal drug to prevent invasive fungal disease in SCT recipients. The primary outcome was the incidence of neurological complications within 7 days of busulfan initiation and was compared between the echinocandin group (patients received prophylactic echinocandin) and nonechinocandin group (patients received prophylactic antifungal drugs other than echinocandin and those without antifungal prophylaxis).ResultsAmong the 59 patients included in this study, the incidence of neurological complications in the echinocandin (n = 26) and nonechinocandin groups (n = 33) was 30.8% and 63.6%, respectively. We observed a negative association between prophylactic echinocandin use and the development of neurological complications after adjusting for the propensity score for receiving prophylactic echinocandins (adjusted odds ratio 0.294, 95% confidence interval 0.090 to 0.959). We observed a lower incidence of neurological complications in the echinocandin group than in the nonechinocandin group.ConclusionOur results suggested that the choice of antifungal prophylaxis is associated with busulfan neurotoxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pediatric Transplantation
医学-小儿科
CiteScore
2.90
自引率
15.40%
发文量
216
审稿时长
3-8 weeks
期刊介绍:
The aim of Pediatric Transplantation is to publish original articles of the highest quality on clinical experience and basic research in transplantation of tissues and solid organs in infants, children and adolescents. The journal seeks to disseminate the latest information widely to all individuals involved in kidney, liver, heart, lung, intestine and stem cell (bone-marrow) transplantation. In addition, the journal publishes focused reviews on topics relevant to pediatric transplantation as well as timely editorial comment on controversial issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


