氟螺利林与 BACE1 的分子动力学和结合能:对干预阿尔茨海默病的意义
IF 1.7
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
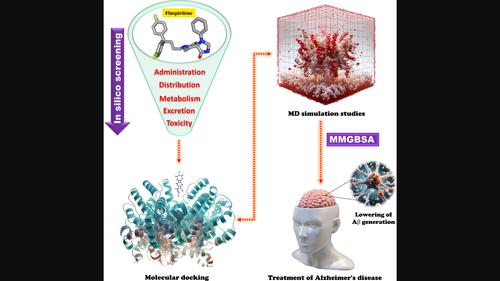
阿尔茨海默病(AD)是一种严重的神经退行性疾病,会导致认知退化、健忘和行为改变,是公共卫生领域的一个重要问题。其发病机制涉及淀粉样蛋白斑块,这凸显了靶向 BACE1 的重要性。本研究将二苯基丁基哌啶 Fluspirilene 作为一种潜在的 BACE1 抑制剂,用于治疗注意力缺失症。对氟司匹林进行了 ADMET 分析。硅学分子对接评估了氟螺环酯与 BACE1 的结合亲和力。重新对接共晶体配体证实了对接过程。为评估对接复合物的稳定性,进行了分子动力学模拟和相关的多方面计算分析。根据 ADMET 分析,氟匹瑞林具有良好的理化和药代动力学特性。硅学分子对接显示了多种 BACE1 相互作用,其结合亲和力为 -9.2 kcal/mol,分子动力学模拟结果证实了氟螺环酯-BACE1 复合物的稳定性。该化合物的药代动力学、分子相互作用和结合能量学显示了它在降低 Aβ 生成和治疗注意力缺失症方面可能的治疗应用。将氟螺环酯作为 BACE1 抑制剂用于 AD 的临床开发需要验证和优化实验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular Dynamics and Binding Energetics of Fluspirilene With BACE1: Implications for Alzheimer's Disease Intervention
Alzheimer's disease (AD) is a serious neurodegenerative disorder that results in cognitive deterioration, amnesia, and alterations in behavior, rendering it a significant issue in public health. The pathogenesis involves amyloid plaques highlighting the importance of targeting BACE1. This study explores fluspirilene, a di‐phenyl‐butyl‐piperidine as a potential BACE1 inhibitor for AD treatment. Fluspirilene was analyzed for ADMET. In silico molecular docking assessed fluspirilene's binding affinity with BACE1. Re‐docking a co‐crystallized ligand confirmed the docking process. Molecular dynamics simulations and related multifaceted computational analyses were conducted to assess the stability of docked complexes. Fluspirilene had good physicochemical and pharmacokinetic characteristics according to ADMET profiling. In silico molecular docking showed multiple BACE1 interactions with a binding affinity of −9.2 kcal/mol and fluspirilene–BACE1 complex stability was confirmed by molecular dynamics simulation results. Possible therapeutic applications in lowering Aβ generation and treating AD are indicated by the compound's pharmacokinetics, molecular interactions, and binding energetics. Validation and optimization of experiments are necessary for the clinical development of fluspirilene as a BACE1 inhibitor for AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


