对血友病患者腺相关病毒壳衍生肽免疫表位特性的调查
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Molecular Therapy-Methods & Clinical Development
Pub Date : 2024-04-02
DOI:10.1016/j.omtm.2024.101245
引用次数: 0
摘要
腺相关病毒(AAV)是治疗单基因疾病的最佳基因载体。然而,针对 AAV 的中和抗体(Nabs)阻碍了其在基因治疗中的广泛应用。在这项研究中,我们从针对 AAV2 的高 Nab 滴度血清中生物合成了被结合抗体(Babs)识别的多肽。我们建立了四种免疫学方法来检测AAV2衍生多肽的免疫表位,包括Bab检测法、Nab检测法、B细胞受体(BCR)检测法和产生免疫球蛋白的B细胞酶联免疫吸附斑(B细胞ELISpot)检测法。利用 89 名 A/B 型血友病患者的血清样本和外周血单核细胞分析了这四种方法确定的表位之间的相关性。作为诱饵,我们使用 AAV 转导模型评估了多肽阻断 AAV2 粒子 Nab 的能力。总之,我们对 AAV2-capsid衍生的多肽免疫表位进行了深入研究,其中涉及 Nab、Bab、BCR 和 B 细胞 ELISpot 检测,提供了在 AAV 介导的基因治疗中克服 Nab 障碍的替代免疫学评估方法和策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
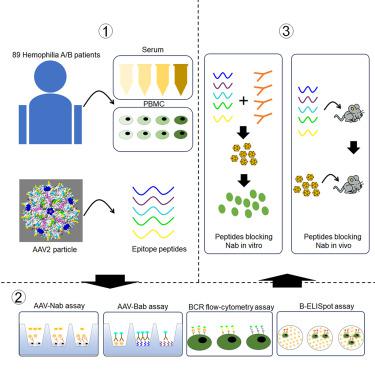
An investigation of the immune epitope properties of adeno-associated virus capsid-derived peptides among hemophilia patients
Adeno-associated virus (AAV) is an optimal gene vector for monogenic disorders. However, neutralizing antibodies (Nabs) against AAV hinder its widespread application in gene therapy. In this study, we biosynthesized peptides recognized by the binding antibodies (Babs) from the sera containing high Nab titers against AAV2. We established four immunological methods to detect immune epitopes of the AAV2-derived peptides, including a Bab assay, Nab assay, B cell receptor (BCR) detecting assay, and immunoglobin-producing B cell enzyme-linked immunosorbent spot (B cell ELISpot) assay. Correlations among the epitopes determined by these four methods were analyzed using the serum samples and peripheral blood mononuclear cells from 89 patients with hemophilia A/B. As decoys, the peptides’ ability to block the Nab of AAV2 particles was assessed using AAV transduction models both and . Overall, we provide insights into AAV2-capsid-derived peptide immune epitopes, involving the Nab, Bab, BCR, and B cell ELISpot assays, offering alternative immunological evaluation approaches and strategies to overcome Nab barriers in AAV-mediated gene therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Therapy-Methods & Clinical Development
Biochemistry, Genetics and Molecular Biology-Molecular Biology
CiteScore
9.90
自引率
4.30%
发文量
163
审稿时长
12 weeks
期刊介绍:
The aim of Molecular Therapy—Methods & Clinical Development is to build upon the success of Molecular Therapy in publishing important peer-reviewed methods and procedures, as well as translational advances in the broad array of fields under the molecular therapy umbrella.
Topics of particular interest within the journal''s scope include:
Gene vector engineering and production,
Methods for targeted genome editing and engineering,
Methods and technology development for cell reprogramming and directed differentiation of pluripotent cells,
Methods for gene and cell vector delivery,
Development of biomaterials and nanoparticles for applications in gene and cell therapy and regenerative medicine,
Analysis of gene and cell vector biodistribution and tracking,
Pharmacology/toxicology studies of new and next-generation vectors,
Methods for cell isolation, engineering, culture, expansion, and transplantation,
Cell processing, storage, and banking for therapeutic application,
Preclinical and QC/QA assay development,
Translational and clinical scale-up and Good Manufacturing procedures and process development,
Clinical protocol development,
Computational and bioinformatic methods for analysis, modeling, or visualization of biological data,
Negotiating the regulatory approval process and obtaining such approval for clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


