CircDiaph3 通过 miR-338-3p/SRSF1 轴加剧 H/R 诱导的心肌细胞凋亡和炎症
摘要
急性心肌梗死(AMI)是最常见的心血管疾病之一,在全球范围内发病率和死亡率都很高。缺氧/复氧(H/R)诱导的心肌细胞损伤是导致急性心肌梗死的主要原因。多项研究表明,环状 RNA 对 AMI 的发病机制有重要影响。在此,我们建立了一个 AMI 小鼠模型来研究 circDiaph3 对心脏功能的影响,并探索 circDiaph3 在 H/R 诱导的心肌细胞损伤中的功能作用及其分子机制。应用生物信息学工具和 RT-qPCR 技术检测 circDiaph3 在人类患者样本、AMI 小鼠心脏组织和 H/R 诱导的 H9C2 细胞中的表达。CCK-8 用于检测细胞活力,而 annexin-V/PI 染色则用于评估细胞凋亡。通过免疫荧光检测心肌活性氧(ROS)水平。用 Western 印迹法检测抗凋亡 Bcl-2、促凋亡 Bax 和裂解-Caspase-3 的蛋白表达。此外,酶联免疫吸附试验(ELISA)用于检测炎性细胞因子的产生。我们还利用生物信息学工具和 RNA 牵引试验来验证 circDiaph3 和 miR-338-3p 之间的相互作用。我们发现,circDiaph3 在 AMI 患者和小鼠以及经 H/R 处理的 H9C2 细胞中高表达。沉默 circDiaph3 可改善体内心肌细胞的凋亡和炎症反应。此外,敲除 cirDiaph3 可减轻 H/R 诱导的细胞凋亡以及 H9C2 细胞中 IL-1β、IL-6 和 TNF-α 等炎症介质的释放。从机理上讲,circDiaph3通过疏导miR-338-3p诱导H/R处理的H9C2细胞发生细胞凋亡和炎症反应。在经 H/R 处理的细胞中过表达 miR-338-3p 能显著逆转 circDiaph3 诱导的效应。值得注意的是,miR-338-3p 可抑制 H/R 处理的 H9C2 细胞中 SRSF1 的表达。而过表达 SRSF1 则会减弱 miR-338-3p 介导的 H/R 处理后细胞凋亡和炎症的缓解作用。综上所述,circDiaph3 通过 miR-338-3p/SRSF1 轴加剧了 H/R 诱导的心肌细胞凋亡和炎症。这些发现表明,circDiaph3/miR-338-3pp/SRSF1 轴可能是治疗 H/R 诱导的心肌损伤的潜在治疗靶点。
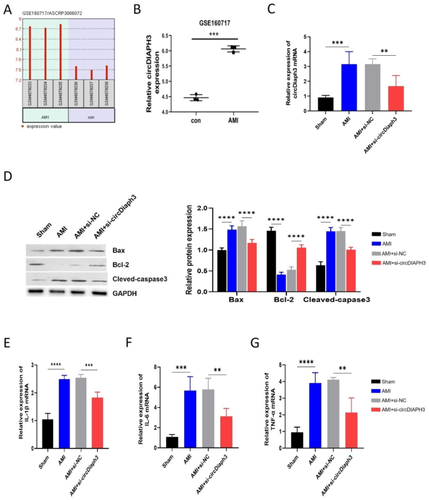
Acute myocardial infarction (AMI) is one of the most prevalent cardiovascular diseases, accounting for a high incidence rate and high mortality worldwide. Hypoxia/reoxygenation (H/R)-induced myocardial cell injury is the main cause of AMI. Several studies have shown that circular RNA contributes significantly to the pathogenesis of AMI. Here, we established an AMI mouse model to investigate the effect of circDiaph3 in cardiac function and explore the functional role of circDiaph3 in H/R-induced cardiomyocyte injury and its molecular mechanism. Bioinformatics tool and RT-qPCR techniques were applied to detect circDiaph3 expression in human patient samples, heart tissues of AMI mice, and H/R-induced H9C2 cells. CCK-8 was used to examine cell viability, while annexin-V/PI staining was used to assess cell apoptosis. Myocardial reactive oxygen species (ROS) levels were detected by immunofluorescence. Western blot was used to detect the protein expression of anti-apoptotic Bcl-2 while pro-apoptotic Bax and cleaved-Caspase-3. Furthermore, ELISA was used to detect inflammatory cytokines production. While bioinformatics tool and RNA pull-down assay were used to verify the interaction between circDiaph3 and miR-338-3p. We found that circDiaph3 expression was high in AMI patients and mice, as well as in H/R-treated H9C2 cells. CircDiaph3 silencing ameliorated apoptosis and inflammatory response of cardiomyocytes in vivo. Moreover, the knockdown of cirDiaph3 mitigated H/R-induced apoptosis and the release of inflammatory mediators like IL-1β, IL-6, and TNF-α in H9C2 cells. Mechanistically, circDiaph3 induced cell apoptosis and inflammatory responses in H/R-treated H9C2 cells by sponging miR-338-3p. Overexpressing miR-338-3p in H/R-treated cells prominently reversed circDiaph3-induced effects. Notably, miR-338-3p inhibited SRSF1 expression in H/R-treated H9C2 cells. While overexpressing SRSF1 abrogated miR-338-3p-mediated alleviation of apoptosis and inflammation after H/R treatment. To summarize, circDiaph3 aggravates H/R-induced cardiomyocyte apoptosis and inflammation through the miR-338-3p/SRSF1 axis. These findings suggest that the circDiaph3/miR-338-3pp/SRSF1 axis could be a potential therapeutic target for treating H/R-induced myocardial injury.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


