从烯胺硫醚快速合成 gem-CF2-2H-Thiophenes
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过烯胺硫醚与含氟碳烯前体的[4+1]环化反应,我们建立了一个简便易行的合成平台,用于构建携带氟原子的 2H-噻吩。这种简单的反应体系在完全无金属的条件下与多种底物兼容。生成的 2H-thiophenes 可进一步进行后期修饰,以产生各种氟取代的杂环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
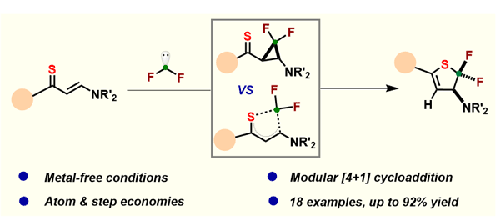
Expedient Synthesis of gem-CF2-2H-Thiophenes from Enaminothiones
An expedient and easy-to-handle synthetic platform has been established for the constructing of 2H-thiophenes carrying fluorine atoms through [4+1] cyclization of enaminothiones with fluorinated carbene precursors. This simple reaction system is well compatible with a wide range of substrates under completely metal-free conditions. The resulting 2H-thiophenes can undergo further late-stage modifications to yield a wide range of fluorine-substituted heterocycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


