脂肪组织巨噬细胞分泌的小细胞外囊泡介导罗格列酮诱导的胰岛素敏感化作用
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
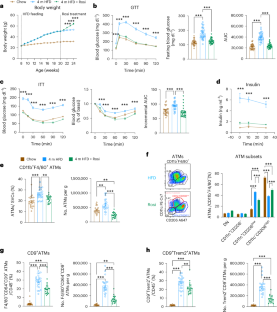
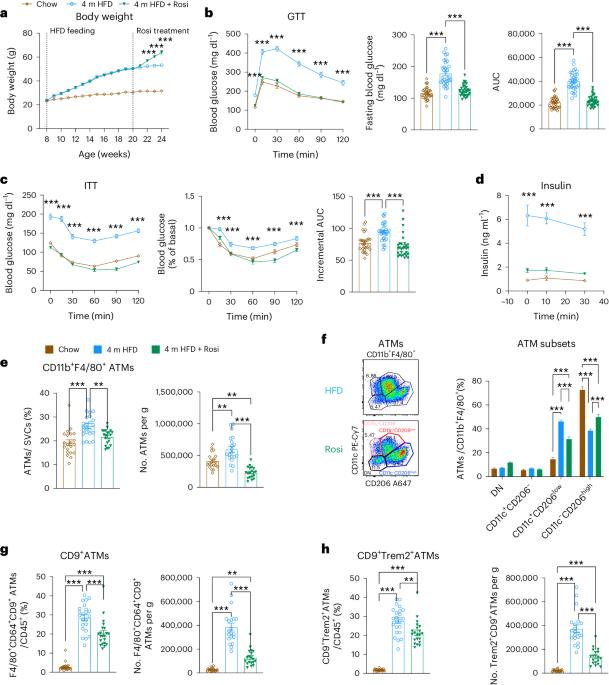
肥胖症在全球范围内不断蔓延,导致代谢性和慢性炎症性疾病。噻唑烷二酮类药物,如罗格列酮(Rosi),是 PPARγ 激动剂,可促进 "类 M2 "脂肪组织巨噬细胞(ATM)极化并导致胰岛素敏感化。已知瘦小鼠的 ATM 衍生小细胞外囊泡(ATM-sEVs)能提高胰岛素敏感性,因此我们评估了经 Rosi 处理的肥胖雄性小鼠的 ATM-sEVs (Rosi-ATM-sEVs)对代谢的影响。我们在此表明,Rosi 可改善葡萄糖和胰岛素耐受性、ATMs 的转录再极化以及 sEV 分泌的增加。服用 Rosi-ATM-sEVs 可以在体内挽救肥胖引起的葡萄糖不耐受和胰岛素敏感性,而不会出现已知的噻唑烷二酮引起的体重增加或血液稀释等不良反应。Rosi-ATM-sEVs 可直接提高脂肪细胞、肌管以及原代小鼠和人类肝细胞的胰岛素敏感性。此外,我们还证明,Rosi-ATM-sEVs 中的 miRNAs(主要是 miR-690)是产生这些有益代谢效应的原因。因此,使用带有特定 miRNA 的 ATM-sEVs 可为诱导胰岛素敏感化提供一条治疗途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Adipose tissue macrophages secrete small extracellular vesicles that mediate rosiglitazone-induced insulin sensitization
The obesity epidemic continues to worsen worldwide, driving metabolic and chronic inflammatory diseases. Thiazolidinediones, such as rosiglitazone (Rosi), are PPARγ agonists that promote ‘M2-like’ adipose tissue macrophage (ATM) polarization and cause insulin sensitization. As ATM-derived small extracellular vesicles (ATM-sEVs) from lean mice are known to increase insulin sensitivity, we assessed the metabolic effects of ATM-sEVs from Rosi-treated obese male mice (Rosi-ATM-sEVs). Here we show that Rosi leads to improved glucose and insulin tolerance, transcriptional repolarization of ATMs and increased sEV secretion. Administration of Rosi-ATM-sEVs rescues obesity-induced glucose intolerance and insulin sensitivity in vivo without the known thiazolidinedione-induced adverse effects of weight gain or haemodilution. Rosi-ATM-sEVs directly increase insulin sensitivity in adipocytes, myotubes and primary mouse and human hepatocytes. Additionally, we demonstrate that the miRNAs within Rosi-ATM-sEVs, primarily miR-690, are responsible for these beneficial metabolic effects. Thus, using ATM-sEVs with specific miRNAs may provide a therapeutic path to induce insulin sensitization. Rohm et al. show that small extracellular vesicles from adipose tissue macrophages from obese rosiglitazone-treated mice ameliorate glucose tolerance and insulin sensitivity in obese mice, while circumventing the adverse effects of rosiglitazone.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


