阻止脂质合成会诱发前列腺癌的 DNA 损伤,并增加 PARP 抑制造成的细胞死亡
IF 6.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
雄激素剥夺疗法(ADT)是治疗前列腺癌的主要方法;然而,ADT总会产生耐药性,导致阉割耐药前列腺癌(CRPC)。由于脂肪酸合成酶(FASN)的过度表达,脂肪酸的从头合成增加,从而使这种酶成为前列腺癌的治疗靶点。抑制 FASN 会导致细胞内神经酰胺和鞘磷脂的含量增加,从而形成 DNA 双链断裂,导致 DNA 损伤和细胞死亡。我们发现,将 FASNi 与聚-ADP 核糖聚合酶(PARP)抑制剂奥拉帕利(奥拉帕利通过阻断 DNA 损伤修复诱导细胞死亡)结合使用,会比单独使用其中一种药物更明显地减少细胞生长。用PARP和FASNi联合治疗的人类CRPC器官组织体积更小,细胞增殖减少,细胞凋亡和坏死增加。这些数据共同表明,以FASN为靶点会损害DNA损伤修复,从而提高PARP抑制剂的疗效,这表明应该探索CRPC的联合疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
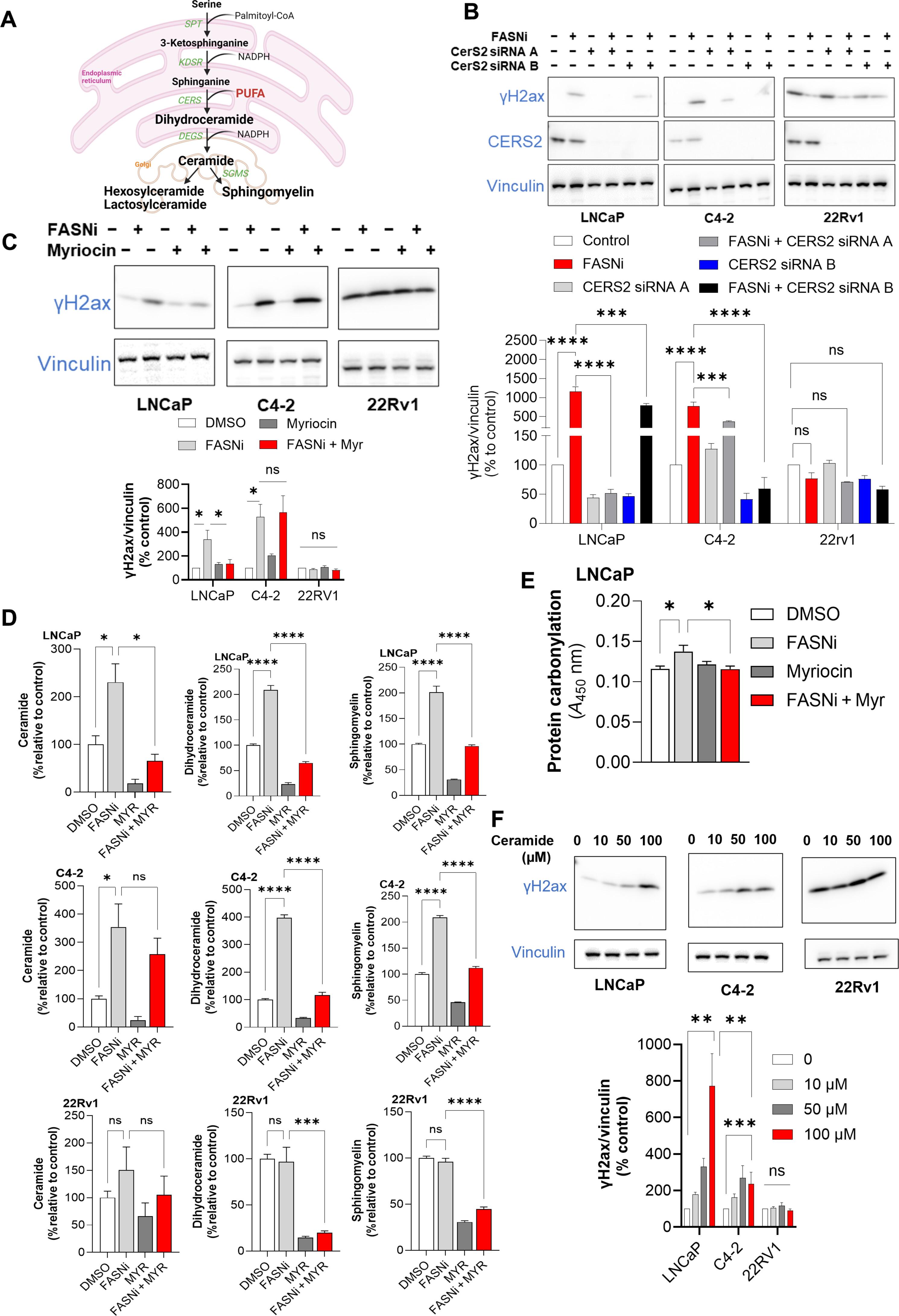
Blocking lipid synthesis induces DNA damage in prostate cancer and increases cell death caused by PARP inhibition
Androgen deprivation therapy (ADT) is the primary treatment for prostate cancer; however, resistance to ADT invariably develops, leading to castration-resistant prostate cancer (CRPC). Prostate cancer progression is marked by increased de novo synthesis of fatty acids due to overexpression of fatty acid synthase (FASN), making this enzyme a therapeutic target for prostate cancer. Inhibition of FASN results in increased intracellular amounts of ceramides and sphingomyelin, leading to DNA damage through the formation of DNA double-strand breaks and cell death. We found that combining a FASNi with the poly-ADP ribose polymerase (PARP) inhibitor olaparib, which induces cell death by blocking DNA damage repair, resulted in a more pronounced reduction in cell growth than that caused by either drug alone. Human CRPC organoids treated with a combination of PARP and FASNi were smaller, had decreased cell proliferation, and showed increased apoptosis and necrosis. Together, these data indicate that targeting FASN increases the therapeutic efficacy of PARP inhibitors by impairing DNA damage repair, suggesting that combination therapies should be explored for CRPC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


