固体收缩的特点:冷却与挤压
IF 2.1
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过实验测定了固体元素和化合物从室温冷却到开氏 0 度或在开氏 298 度用机械压缩到相同体积的收缩率。我们发现,冷压缩的能量成本平均比机械压缩的能量成本高出两个数量级。这一事实是由不同的收缩机制造成的:压制直接减小了原子间距离,冷却主要减小了原子谐波振动的振幅,而造成热收缩的振动能量的非谐波部分非常小,约为 1%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
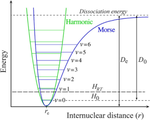
Features of contraction of solids: cooling vs pressing
Contraction of solid elements and compounds by cooling from room temperature to 0 K or by mechanical pressing to the same volume at 298 K is experimentally determined. We found that the energy cost of cold compression exceeds the energy of mechanical compression on average by two orders of magnitude. This fact is caused by the different mechanisms of contraction: pressing directly reduces interatomic distances, cooling mainly reduces the amplitudes of harmonic vibrations of atoms, whereas the anharmonic part of the vibration energy, responsible for the thermal contraction, is very small, ca. 1%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structural Chemistry
化学-化学综合
CiteScore
3.80
自引率
11.80%
发文量
227
审稿时长
3.7 months
期刊介绍:
Structural Chemistry is an international forum for the publication of peer-reviewed original research papers that cover the condensed and gaseous states of matter and involve numerous techniques for the determination of structure and energetics, their results, and the conclusions derived from these studies. The journal overcomes the unnatural separation in the current literature among the areas of structure determination, energetics, and applications, as well as builds a bridge to other chemical disciplines. Ist comprehensive coverage encompasses broad discussion of results, observation of relationships among various properties, and the description and application of structure and energy information in all domains of chemistry.
We welcome the broadest range of accounts of research in structural chemistry involving the discussion of methodologies and structures,experimental, theoretical, and computational, and their combinations. We encourage discussions of structural information collected for their chemicaland biological significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


