通过光氧化诱导的氢转移/Giese Addition/ Dearomative Cyclization/Protonation Cascade†,以完全原子经济的方式获得螺环骨架
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报告了一种完全原子与无活化苯基和其他杂芳基环的高步经济级联脱芳烃反应,以获得具有广泛底物范围的乙醚苯胺螺内酰胺。该方案涉及光氧化诱导的氢转移/吉斯加成/脱芳香环化/质子化级联反应,可将 N-甲基苯胺的 C(sp3)-H 键的两个片段引入 N-苄基丙烯酰胺。有趣的是,吡啶环的脱芳香产物可以以完全原子经济的方式进一步转化为五环生物碱骨架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
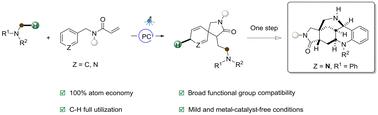
Fully atom-economic access to spiro-cyclic skeletons through photoredox-induced hydrogen transfer/Giese addition/dearomative cyclization/protonation cascade†
Herein, we report a fully atom-economic and highly step-economic cascade dearomatization with non-activated phenyl and other hetero-aryl rings for access to aniline-tethered spiro-lactams with broad substrate scopes. This protocol involves a photoredox-induced hydrogen transfer/Giese addition/dearomative cyclization/protonation cascade and enables the introduction of two moieties of a C(sp3)–H bond of N-methylaniline into N-benzyl-acrylamide. Interestingly, dearomative products of the pyridine ring can be further transformed into pentacyclic alkaloid skeletons in a fully atom-economic fashion again.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


