SDCBP 可调节结直肠癌的肿瘤微环境、肿瘤进展和抗 PD1 的疗效。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
抗程序性细胞死亡 1(aPD1)疗法对结直肠癌(CRC)患者的治疗效果有限。Syndecan结合蛋白(SDCBP)编码一种含PDZ结构域的蛋白,对细胞粘附、迁移和信号转导等细胞过程至关重要。在此,我们研究了 SDCBP 对 CRC 肿瘤进展、免疫疗法和肿瘤微环境(TME)的影响。SDCBP的高表达与免疫疗法无反应有关,并与CRC患者较差的无病生存期(DFS)相关。在小鼠异种移植模型和肝转移模型中,通过转染 shRNA 或使用其抑制剂吡啶硫酮锌(ZnPT)抑制 SDCBP 会阻碍增殖和转移,同时提高 aPD1 治疗的疗效。ZnPT治疗后,CRC的TME发生了显著变化,M2巨噬细胞数量减少,M1巨噬细胞比例增加。CRC 细胞和巨噬细胞的共培养系统证明,沉默 SDCBP 能促进 M2 巨噬细胞极化为 M1。SDCBP 促进了 CRC 的增殖、转移和免疫治疗抵抗。因此,ZnPT 是一种有效的 SDCBP 抑制剂,具有与 aPD1 联用以提高免疫疗法疗效的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
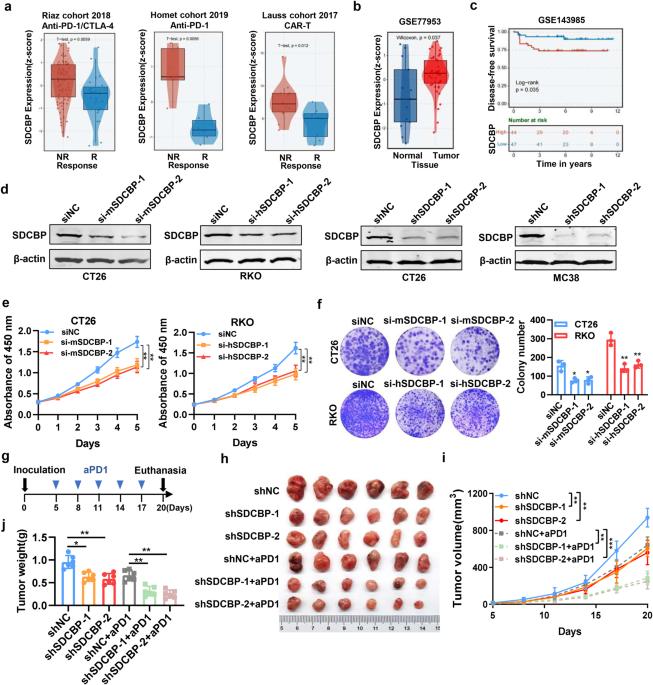
SDCBP modulates tumor microenvironment, tumor progression and anti-PD1 efficacy in colorectal cancer
Anti-programmed cell death 1 (aPD1) therapy has yielded limited success in patients with colorectal cancer (CRC). Syndecan binding protein (SDCBP), encodes a PDZ domain-containing protein that is essential for cellular processes, including cell adhesion, migration, and signal transduction. Here, we investigated the effect of SDCBP on tumor progression, immunotherapy, and the tumor microenvironment (TME) in CRC. High expression of SDCBP is associated with non-response to immunotherapy and correlated with poorer disease-free survival (DFS) in CRC patients. Inhibiting SDCBP by transfecting shRNA or using its inhibitor zinc pyrithione (ZnPT) hindered proliferation and metastasis while enhancing the efficacy of aPD1 treatment in a mouse xenograft model and liver metastasis model. The TME of CRC was significantly altered following ZnPT treatment characterized by a reduced amount of M2 macrophages and a heightened percentage of M1 macrophages. The co-culture system of CRC cells and macrophages provided evidence that SDCBP silencing promoted the repolarisation of M2 macrophages into M1. SDCBP promotes the proliferation, metastasis, and immunotherapy resistance of CRC. Thus, ZnPT represents an effective SDCBP inhibitor and exhibits considerable potential for combination with aPD1 to enhance immunotherapy efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


