巨细胞病毒感染星形胶质细胞接种小鼠后产生胶质母细胞瘤。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
越来越多的证据表明,人类巨细胞病毒(HCMV)是一种潜在的致癌病毒。在多形性胶质母细胞瘤(GB)中检测到了 HCMV。在此,我们首次通过实验证明,CMV 诱导的胶质母细胞瘤细胞(CEGBCs)具有类似胶质母细胞瘤的特征,可在异种移植小鼠体内形成胶质母细胞瘤。除了已经报道过的致癌 HCMV-DB 株系外,我们还从 GB 组织中分离出了三种 HCMV 临床株系,它们能使 HAs 向 CEGBCs 转化,并从 CEGBCs 中生成球体,从而导致异种移植小鼠出现胶质母细胞瘤样肿瘤。这些肿瘤主要在浸润部位呈 nestin 阳性,周围是 GFAP 阳性的反应性星形胶质细胞。肿瘤中表皮生长因子受体(EGFR)和 cMet 基因扩增证实了胶质母细胞瘤的免疫组化表型,同时还检测到 HCMV IE 和 UL69 基因及蛋白。我们的研究结果符合 HCMV 诱导的胶质母细胞瘤体内肿瘤发生模型,这将为新的治疗方法打开大门,并评估抗 HCMV 治疗和免疫疗法在抗击预后不良的 GB 方面的效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
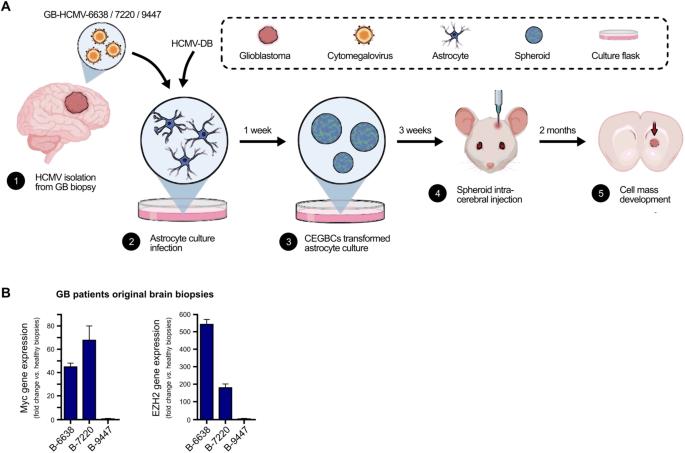
Generation of glioblastoma in mice engrafted with human cytomegalovirus-infected astrocytes
Mounting evidence is identifying human cytomegalovirus (HCMV) as a potential oncogenic virus. HCMV has been detected in glioblastoma multiforme (GB). Herewith, we present the first experimental evidence for the generation of CMV-Elicited Glioblastoma Cells (CEGBCs) possessing glioblastoma-like traits that lead to the formation of glioblastoma in orthotopically xenografted mice. In addition to the already reported oncogenic HCMV-DB strain, we isolated three HCMV clinical strains from GB tissues that transformed HAs toward CEGBCs and generated spheroids from CEGBCs that resulted in the appearance of glioblastoma-like tumors in xenografted mice. These tumors were nestin-positive mostly in the invasive part surrounded by GFAP-positive reactive astrocytes. The glioblastoma immunohistochemistry phenotype was confirmed by EGFR and cMet gene amplification in the tumor parallel to the detection of HCMV IE and UL69 genes and proteins. Our results fit with an HCMV-induced glioblastoma model of oncogenesis in vivo which will open the door to new therapeutic approaches and assess the anti-HCMV treatment as well as immunotherapy in fighting GB which is characterized by poor prognosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


