(S)-焦谷氨酸的实用合成
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过在无溶剂条件下对(S)-谷氨酸进行热循环脱水,并在加热(220°C)过程中不断搅拌以确保熔体的均匀性,开发出了一种简单快速的(S)-焦谷氨酸合成方法。反应进行到气泡消失为止,时间不超过 5 分钟。然后立即停止加热,冷却反应容器以防止降解和外消旋化。(S)-焦谷氨酸的产率为 70%,对映体比例为 97:3。在水溶液中用微波加热进行同样的反应,得到的外消旋产物收率较低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
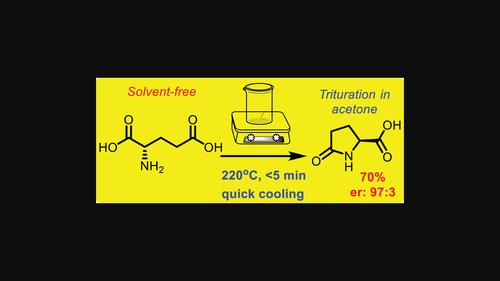
Practical synthesis of (S)-pyroglutamic acid
A simple and rapid synthesis of (S)-pyroglutamic acid was developed by the thermal cyclodehydration of (S)-glutamic acid under solvent-free conditions, with continuous swirling during heating (220°C) to ensure homogeneity of the melt. The reaction proceeds until the bubbles disappear, not exceeding 5 min. The heating immediately ceases, then cooling the reaction vessel to prevent degradation and racemization. (S)-pyroglutamic acid is obtained in 70% yield, and the enantiomeric ratio is 97:3. The same reaction under microwave heating in an aqueous solution afforded a low yield of racemic product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


