避免使用乙酰氯,在 TfOH 催化下轻松实现 β-酰氨基酮的一锅合成
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种在 TfOH 催化下从醛、酮和腈合成具有生物活性的 β-酰氨基酮的高效方法,避免了乙酰氯的使用。该反应通过一个连续的醛醇反应进行,然后是腈的亲核反应和腈的水解反应。这种串联工艺的诱人之处在于反应条件温和、原子经济性高、底物范围广(产率为 51-87%)、反应规模为克级以及操作简便。本文章由计算机程序翻译,如有差异,请以英文原文为准。
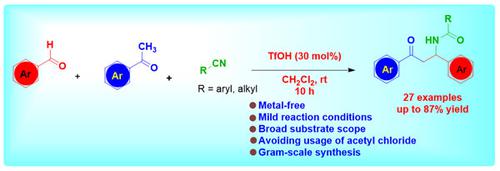
TfOH-Catalyzed Facile Access for One-Pot Synthesis of β-Acylamino Ketones by Avoiding the Usage of Acetyl Chloride
We have developed a TfOH-catalyzed, highly efficient protocol for the synthesis of biologically active β-acylamino ketones from aldehyde, ketone, and nitrile by avoiding the use of acetyl chloride. The reaction proceeds through a sequential aldol reaction followed by a nucleophilic attack of nitrile and hydrolysis of nitrile in one pot. The attractive features of this tandem process are mild reaction conditions, high atom economy, broad substrate scope with 51–87% yield, gram-scale reaction, and ease of operation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


